185361
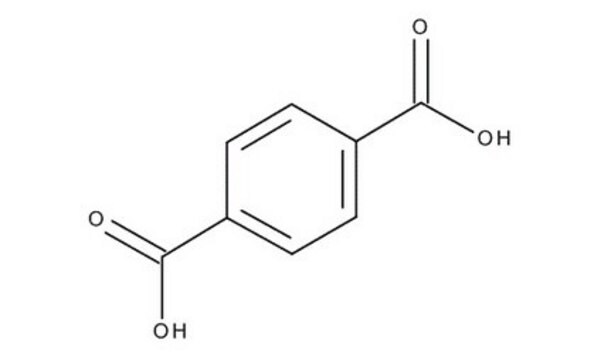
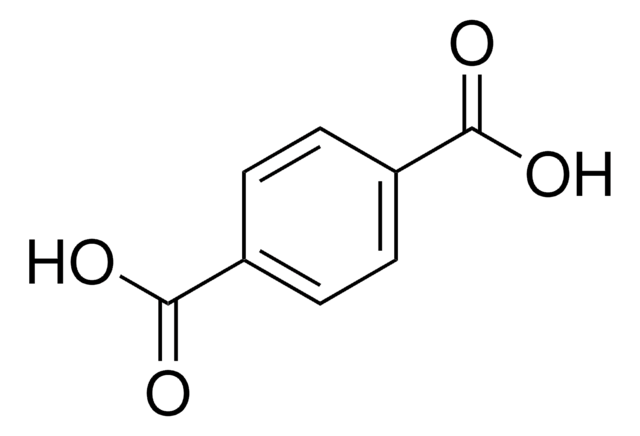
Terephthalic acid
98%
Synonyme(s) :
Benzene-1,4-dicarboxylic acid
About This Item
Produits recommandés
Pression de vapeur
<0.01 mmHg ( 20 °C)
Pureté
98%
Forme
powder
Température d'inflammation spontanée
925 °F
Caractéristiques du produit alternatif plus écologique
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Pf
>300 °C (lit.)
Solubilité
water: ~0.017 g/L at 25 °C
Densité
1.58 g/cm3 at 25 °C
Autre catégorie plus écologique
Chaîne SMILES
OC(=O)c1ccc(cc1)C(O)=O
InChI
1S/C8H6O4/c9-7(10)5-1-2-6(4-3-5)8(11)12/h1-4H,(H,9,10)(H,11,12)
Clé InChI
KKEYFWRCBNTPAC-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Catégories apparentées
Description générale
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for energy efficiency. Find details here.
Application
- As a monomer in the synthesis of poly(butylene terephthalate) (PBT), a type of polyester, that is used in various fields including Automotive components, textile Industry, packaging materials, electrical and electronic components.
- As an organic ligand in the synthesis of the cobalt(II) metal–organic framework (MOFs), which finds applications in electrochemical energy storage, catalysis, optoelectronics, and water treatment.
- Terephthalic acid (TPA) can be synthesized from bio-based materials for a variety of applications, which include the production of polyester fiber, non-fiber field, PET bottles, synthetic perfumes and medicines.
- Terephthalic acid is used as a linker molecule in the preparation of metal-organic frameworks (MOFs).
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique