Y3125
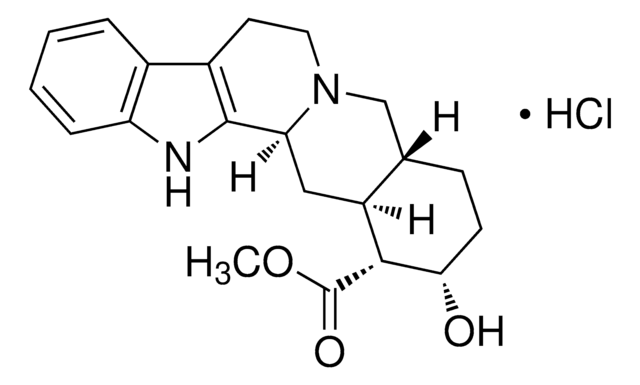
Yohimbine hydrochloride
≥98% (HPLC), powder, α2-adrenergic receptor antagonist
Synonym(s):
17-Hydroxyyohimban-16-carboxylic acid methyl ester hydrochloride
About This Item
Recommended Products
Product Name
Yohimbine hydrochloride, ≥98% (HPLC), powder
Quality Level
assay
≥98% (HPLC)
form
powder
color
white to off-white
mp
288-290 °C (dec.) (lit.)
solubility
H2O: 10 mg/mL
SMILES string
Cl.COC(=O)[C@H]1[C@@H](O)CCC2CN3CCc4c([nH]c5ccccc45)C3CC12
InChI
1S/C21H26N2O3.ClH/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24;/h2-5,12,15,17-19,22,24H,6-11H2,1H3;1H/t12-,15-,17-,18-,19+;/m0./s1
InChI key
PIPZGJSEDRMUAW-VJDCAHTMSA-N
Gene Information
human ... ADRA2A(150) , ADRA2B(151) , ADRA2C(152)
Looking for similar products? Visit Product Comparison Guide
Application
- used to study its in vitro antiviral, antibacterial and antifungal activities and cytotoxicity
- used as a tyramine receptor blocker to oppose the effects of increased tyramine in relation with flight initiation and maintenance deficits in experimental flies
- used alone and as well as with brimonidine to study the neuroprotective effect of brimonidine in the presence of glutamate-induced neurotoxicity, oxidative stress and hypoxia on in vitro cultures of purified rat retinal ganglion cells
Biochem/physiol Actions
Features and Benefits
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 2 Oral
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
α2-Adrenoceptors
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service