C3144
Coenzyme A sodium salt hydrate
cofactor for acyl transfer
Synonym(s):
CoA Na2
About This Item
Recommended Products
description
cofactor for acyl transfer
Quality Level
assay
≥85% (spectrophotometric assay)
form
powder
solubility
H2O: soluble 50 mg/mL, clear, colorless to faintly yellow
storage temp.
−20°C
SMILES string
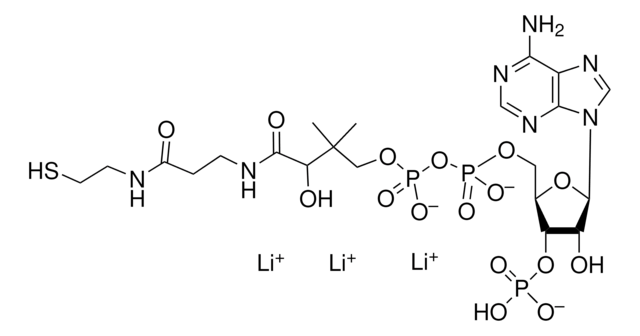
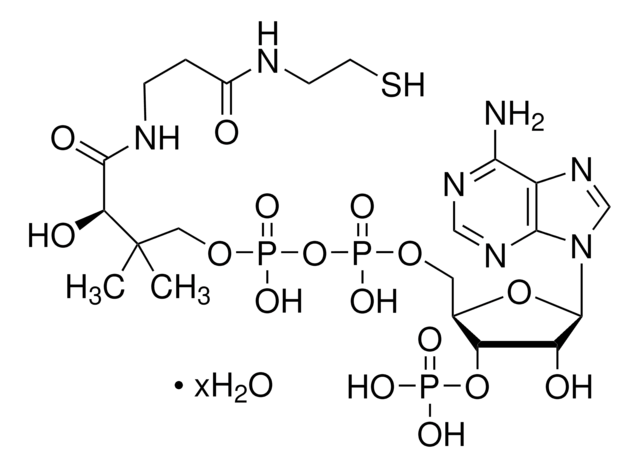
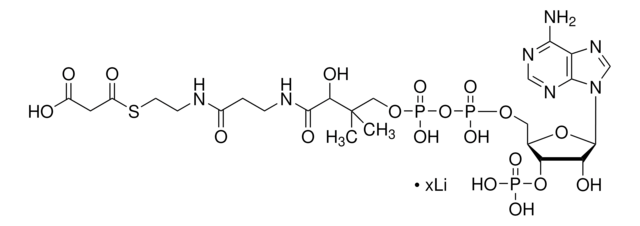
[Na+].CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)([O-])=O)n2cnc3c(N)ncnc23)C(O)C(=O)NCCC(=O)NCCS
InChI key
SYTRWOCXZXQBPW-CLVRNSBASA-M
Looking for similar products? Visit Product Comparison Guide
General description
Application
- gylcerolipid biosynthesis in porcine adipose tissue
- an assay to measure the level of Alpha-methylacyl-CoA racemase (AMACR) in human blood samples using a nanoparticle electrochemical biosensor
- chloramphenicol acetyltransferase (CAT) assay
- the synthesis of palmitoyl-CoA, which is required for palmitoylation and activation of proteins for regulated membrane fusion
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Get to know the Tricarboxylic acid (TCA) cycle to better inform your research in biochemistry, metabolomics, or related fields concerned with this metabolic pathway and its enzymes, by-products, or intermediates.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service