A5006
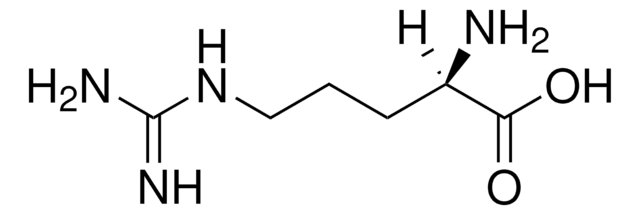
L-Arginine
≥98%
Synonym(s):
(S)-2-Amino-5-guanidinopentanoic acid
About This Item
Recommended Products
product name
L-Arginine, reagent grade, ≥98%
grade
reagent grade
Quality Level
assay
≥98%
form
powder
color
white
mp
222 °C (dec.) (lit.)
solubility
H2O: 50 mg/mL
application(s)
cell analysis
peptide synthesis
SMILES string
N[C@@H](CCCNC(N)=N)C(O)=O
InChI
1S/C6H14N4O2/c7-4(5(11)12)2-1-3-10-6(8)9/h4H,1-3,7H2,(H,11,12)(H4,8,9,10)/t4-/m0/s1
InChI key
ODKSFYDXXFIFQN-BYPYZUCNSA-N
Gene Information
human ... NOS1(4842) , NOS2(4843)
rat ... Ppm1a(24666)
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 1
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
fructose 1,6-bisphosphate in ice
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service