T0625
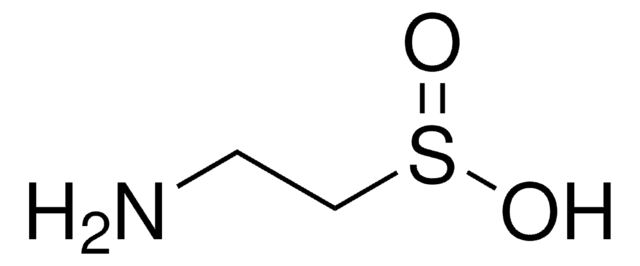
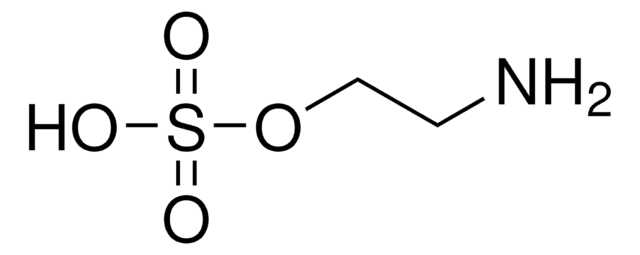
Taurine
≥99%
Synonym(s):
2-Aminoethanesulfonic acid
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
NH2CH2CH2SO3H
CAS Number:
Molecular Weight:
125.15
Beilstein/REAXYS Number:
1751215
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Product Name
Taurine, ≥99%
biological source
synthetic
assay
≥99%
form
powder
color
white
mp
>300 °C (lit.)
application(s)
cell analysis
storage temp.
room temp
SMILES string
NCCS(O)(=O)=O
InChI
1S/C2H7NO3S/c3-1-2-7(4,5)6/h1-3H2,(H,4,5,6)
InChI key
XOAAWQZATWQOTB-UHFFFAOYSA-N
Gene Information
human ... GRIN1(2902)
rat ... Ppm1a(24666)
Looking for similar products? Visit Product Comparison Guide
General description
Taurine (2-aminoethanesulphonic acid) is predominantly found in the retina and heart and is also found in the brain, intestine, skeletal muscles and kidneys.
Application
Taurine has been used for the isolation and growth of taurine-utilizing purple non-sulfur bacteria and in phototrophic growth experiments.
Biochem/physiol Actions
Non-selective endogenous agonist at glycine receptors.
Non-selective endogenous agonist at glycine receptors. Conditionally essential sulfonated amino acid which modulates apoptosis in some cells; functions in many metabolic activities; a product of methionine and cysteine metabolism.
Taurine modulates the concentration of intracellular calcium, protects against ischemia-reperfusion injury and possesses blood pressure-lowering properties. It also has a role in bile formation and fat digestion. Deficiency of taurine is associated with anxiety, hyperactivity, epilepsy and depression.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Phototrophic utilization of taurine by the purple nonsulfur bacteria Rhodopseudomonas palustris and Rhodobacter sphaeroides.
Novak RT
Microbiology, 150(Pt 6), 1881-1891 (2004)
The potential health benefits of taurine in cardiovascular disease.
Xu YJ
Experimental and Clinical Cardiology, 13(2), 57-65 (2008)
M Axelson et al.
Hepatology (Baltimore, Md.), 31(6), 1305-1312 (2000-05-29)
The biosynthesis of bile acids by primary cultures of normal human hepatocytes has been investigated. A general and sensitive method for the isolation and analysis of sterols and bile acids was used, based on anion exchange chromatography and gas chromatography-mass
Deniz Tasdemir et al.
Bioorganic & medicinal chemistry, 15(21), 6834-6845 (2007-09-04)
The type II fatty acid pathway (FAS-II) is a validated target for antimicrobial drug discovery. An activity-guided isolation procedure based on Plasmodium falciparum enoyl-ACP reductase (PfFabI) enzyme inhibition assay on the n-hexane-, the CHCl(3-) and the aq MeOH extracts of
Felizia K Voss et al.
Science (New York, N.Y.), 344(6184), 634-638 (2014-05-03)
Regulation of cell volume is critical for many cellular and organismal functions, yet the molecular identity of a key player, the volume-regulated anion channel VRAC, has remained unknown. A genome-wide small interfering RNA screen in mammalian cells identified LRRC8A as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service