251275
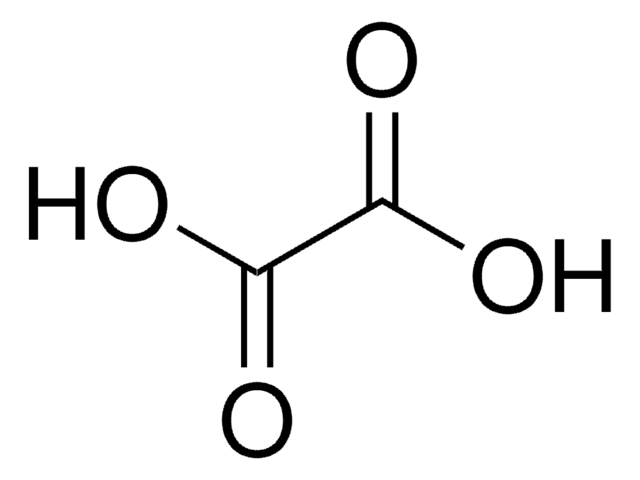
Citric acid
ACS reagent, ≥99.5%
Synonym(s):
2-Hydroxy-1,2,3-propanetricarboxylic acid, 2-Hydroxypropan-1,2,3-tricarboxylic acid
About This Item
Recommended Products
grade
ACS reagent
Quality Level
assay
≥99.5%
form
crystals
expl. lim.
8 %, 65 °F
impurities
Substances carbonizable by hot sulfuric acid, passes test
ign. residue
≤0.02%
pKa
(1) 3.13, (2) 4.76, (3) 6.4
mp
153-159 °C (lit.)
anion traces
chloride (Cl-): ≤0.001%
oxalate (C2O42-): passes test (limit about 0.003%)
phosphate (PO43-): ≤0.001%
sulfate (SO42-): ≤0.002%
cation traces
Fe: ≤3 ppm
Pb: ≤2 ppm
functional group
carboxylic acid
hydroxyl
SMILES string
OC(=O)CC(O)(CC(O)=O)C(O)=O
InChI
1S/C6H8O7/c7-3(8)1-6(13,5(11)12)2-4(9)10/h13H,1-2H2,(H,7,8)(H,9,10)(H,11,12)
InChI key
KRKNYBCHXYNGOX-UHFFFAOYSA-N
Gene Information
human ... SRC(6714)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Phosphate citrate buffer for use in enzyme-linked immunosorbent assay.
- Citrate-stabilized ceria aqueous sol, which was employed in the synthesis of cerium oxide nanoparticles.
- Citric acid-Na2HPO4-buffered stock solution for use in the determination of fecal urease activity.
- Anticoagulant citrate dextrose solution A (ACD-A), which is employed during the isolation of blood-derived endothelial progenitor cells.
Citric acid has also been used:
- In a novel process which allows controlling of the particle size during the synthesis of palladium cuboctahedrons.
- To prepare citric acid-derived carbon nanodots (CNDs) by bottom-up carbonization method.
- As a bi-component chelating agent for the synthesis of Li4Ti5O12 (lithium titanate oxide) by a novel sol–gel method.
Other Notes
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
A complete workflow for the intact and middle-up mass analysis of reduced and non-reduced monoclonal antibodies based on SEC-MS with sample preparation by protein-A affinity clean-up.
Related Content
Step-by-step reversed phase UHPLC-MS workflow for middle-up mass analysis of an immunoglobulin G antibody, consisting of antibody purification, IdeS proteolysis and reduction, mass spectrometer calibration, mAb quantification, and a system suitability test.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service