14007
Potassium titanium oxide oxalate dihydrate
Synonym(s):
Dipotassium oxodioxalatotitanate(IV) dihydrate, Oxotitanium potassium ethanedioate hydrate (1:2:2:2)
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
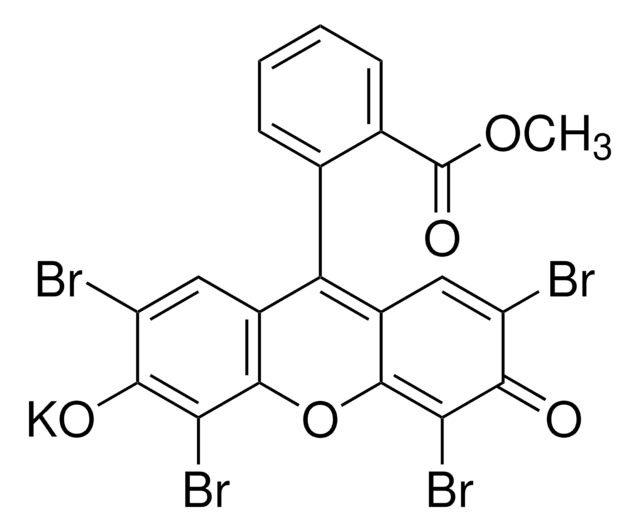
Empirical Formula (Hill Notation):
C4K2O9Ti · 2H2O
CAS Number:
Molecular Weight:
354.13
EC Number:
MDL number:
UNSPSC Code:
12161600
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
form
solid
Quality Level
reaction suitability
core: titanium
reagent type: catalyst
SMILES string
O.O.[K+].[K+].[O-]C(=O)C(=O)O[Ti](=O)OC(=O)C([O-])=O
InChI
1S/2C2H2O4.2K.2H2O.O.Ti/c2*3-1(4)2(5)6;;;;;;/h2*(H,3,4)(H,5,6);;;2*1H2;;/q;;2*+1;;;;+2/p-4
InChI key
PGGRHIGITIPOBF-UHFFFAOYSA-J
General description
Potassium titanium oxide oxalate dihydrate is used as a catalyst in organic synthesis because of its excellent water solubility. It can be easily separated from the reaction mixture after the completion of the reaction.
Application
Potassium titanium oxide oxalate (PTO) dihydrate can be used as:
PTO can be used as a precursor to synthesize titanium dioxide (TiO2) nanoparticles.
- A catalyst to synthesize chromene derivatives via three-component condensation reaction of aromatic aldehydes, malononitrile, and resorcinol/ naphthol.
- An electrolyte to functionalize the surface of aluminum alloy with TiO2 layer by plasma electrolytic oxidation (PEO).
PTO can be used as a precursor to synthesize titanium dioxide (TiO2) nanoparticles.
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Electrorheological properties of suspensions of hollow globular titanium oxide/polypyrrole particles.
Sedlacik, M.
Colloid and Polymer Science, 290(1), 41-48 (2012)
Sub-100? C solution processed amorphous titania nanowire thin films for high-performance perovskite solar cells.
Wu W Q, et al.
Journal of Power Sources, 329, 17-22 (2016)
Formation of multi-functional TiO2 surfaces on AA2024 alloy using plasma electrolytic oxidation
Ignjatovic, S, et al.
Applied Surface Science, 544, 148875-148875 (2021)
Rusen Zou et al.
iScience, 24(2), 102094-102094 (2021-03-23)
Microbial electrosynthesis system (MES) has recently been shown to be a promising alternative way for realizing in situ and energy-saving synthesis of hydrogen peroxide (H2O2). Although promising, the scaling-up feasibility of such a process is rarely reported. In this study
Ultrasonic synthesis of substituted chromenes by utilizing potassium titanium oxalate
Manake AP, et al.
Materials Today: Proceedings, 26, 3487-3491 (2020)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service