All Photos(1)
About This Item
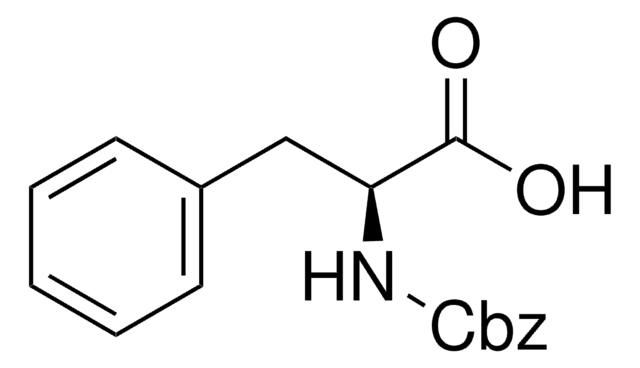
Linear Formula:
C6H5CH2OOCNHCH2COOH
CAS Number:
Molecular Weight:
209.20
Beilstein/REAXYS Number:
526877
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
99%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
color
beige
mp
118-122 °C (lit.)
application(s)
peptide synthesis
SMILES string
OC(=O)CNC(=O)OCc1ccccc1
InChI
1S/C10H11NO4/c12-9(13)6-11-10(14)15-7-8-4-2-1-3-5-8/h1-5H,6-7H2,(H,11,14)(H,12,13)
InChI key
CJUMAFVKTCBCJK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Z-Gly-OH, also known as N-benzyloxycarbonylglycine, is an amino acid widely used in solution phase peptide synthesis.
Application
Z-Gly-OH is a versatile reagent that can be used to synthesize a variety of compounds such as:
- glycine-derived peptides like Z-Gly-DL-Ala-OBzl and Z-Gly-L-Ala-OBzl
- glycine N-substituted amides such as glycine-N-methylamide hydrochloride and glycine-N-isopropylamide hydrochloride
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Studies on Separation of Amino Acids and Related Compounds. V. A Racemization Test in Peptide Synthesis by the Use of an Amino Acid Analyzer
Bulletin of the Chemical Society of Japan, 44, 3391-3395 (1971)
Duality of mechanism in the tetramethylfluoroformamidinium hexafluorophosphate-mediated synthesis of N-benzyloxycarbonylamino acid fluorides.
R Fiammengo et al.
The Journal of organic chemistry, 66(17), 5905-5910 (2001-08-21)
G K Scriba et al.
The Journal of pharmacy and pharmacology, 51(5), 549-553 (1999-07-20)
Glycine, which has weak anticonvulsant properties, has been shown to potentiate the activity of several antiepileptic drugs but not phenytoin. Recently, studies have shown that N-(benzyloxycarbonyl)glycine (Z-glycine) antagonized seizures more than glycine in addition to possessing activity in the maximal
D M Lambert et al.
Neuroreport, 5(7), 777-780 (1994-03-21)
Although glycine does not cross easily the blood-brain barrier, it exhibits at very high doses (10-40 mmol kg-1) a modest anticonvulsant activity. In this study, carbamate derivatives--N-benzyloxycarbonylglycine (Z-glycine) and N,tert-butoxycarbonylglycine (Boc-glycine)--have been compared with glycine. Z-glycine (1 mmol kg-1), but
David J Merkler et al.
Bioorganic & medicinal chemistry, 16(23), 10061-10074 (2008-10-28)
Peptidyl alpha-hydroxylating monooxygenase (PHM) functions in vivo towards the biosynthesis of alpha-amidated peptide hormones in mammals and insects. PHM is a potential target for the development of inhibitors as drugs for the treatment of human disease and as insecticides for
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service