439606
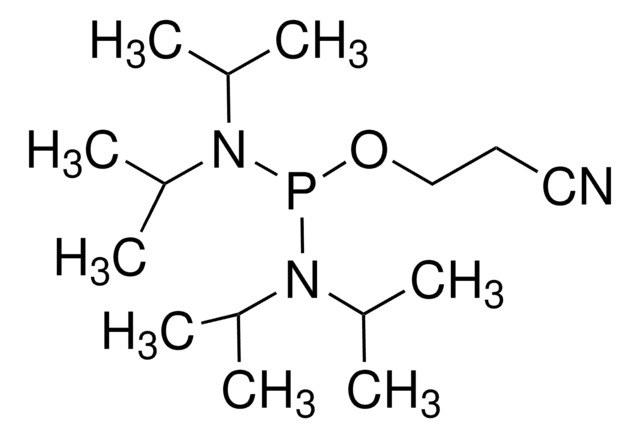
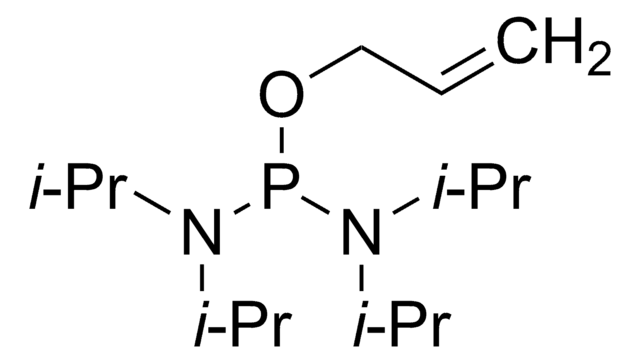
Diallyl N,N-diisopropylphosphoramidite
Synonym(s):
N,N-Bis(1-methylethyl)phosphoramidous acid di-2-propenyl ester, Diallyloxy(diisopropylamino)phosphine
About This Item
Recommended Products
form
liquid
Quality Level
refractive index
n20/D 1.456 (lit.)
bp
130 °C (lit.)
density
0.928 g/mL at 25 °C (lit.)
functional group
amine
storage temp.
2-8°C
SMILES string
CC(C)N(C(C)C)P(OCC=C)OCC=C
InChI
1S/C12H24NO2P/c1-7-9-14-16(15-10-8-2)13(11(3)4)12(5)6/h7-8,11-12H,1-2,9-10H2,3-6H3
InChI key
QBLCHHSGJTUNSJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Preparation of peptide substrates.
- Preparation of rhodamine dyes with phosphorylated CH2OH sites.
- Synthesis of 3-[4-(bis-allyloxy-phosphoryloxy)-2(R)-hydroxy-3,3-dimethyl-butyrylamino]-thiopropionic acid-S-propylester.
- Selective orally active S1P1 agonists
- Water-soluble prodrugs of triazole CS-758 with antifungal activity
- Fostriecin analogs as antitumor agents
Reactant involved in:
- Phosphitylation of alcohols
- Deallylation for the synthesis of nucleoside phosphoramidite
- Posphitylation and stereoselective Pudovik rearrangement
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
89.6 °F - closed cup
flash_point_c
32 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service