366331
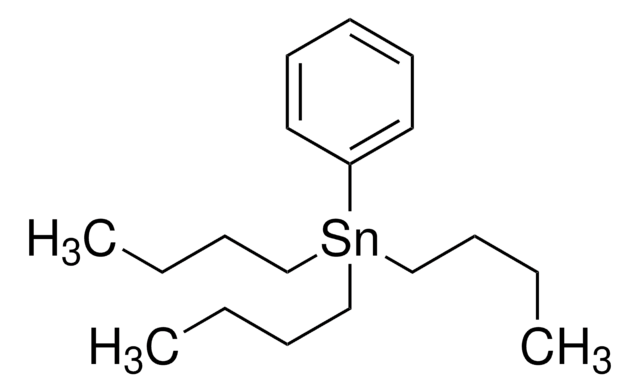
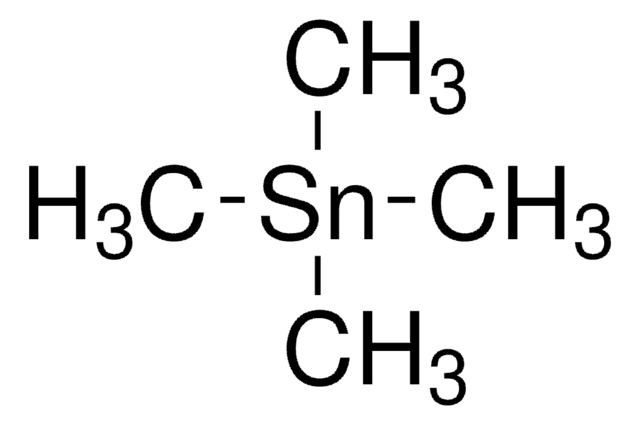
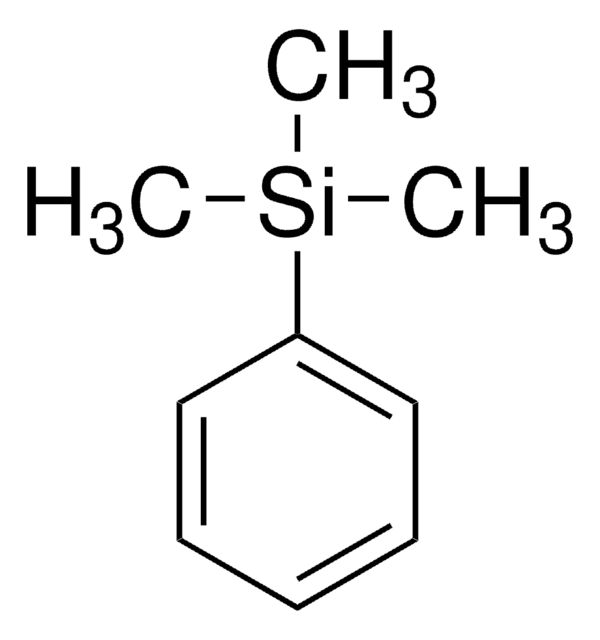
Trimethyl(phenyl)tin
98%
Synonym(s):
Phenyltrimethylstannane, Phenyltrimethyltin, Trimethylmonophenyltin, Trimethylphenylstannane, Trimethylstannylbenzene
About This Item
Recommended Products
Quality Level
assay
98%
form
liquid
reaction suitability
core: tin
refractive index
n20/D 1.5357 (lit.)
bp
88 °C/16 mmHg (lit.)
mp
−51 °C (lit.)
density
1.327 g/mL at 25 °C (lit.)
SMILES string
C[Sn](C)(C)c1ccccc1
InChI
1S/C6H5.3CH3.Sn/c1-2-4-6-5-3-1;;;;/h1-5H;3*1H3;
InChI key
COHOGNZHAUOXPA-UHFFFAOYSA-N
General description
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 1 Dermal - Acute Tox. 1 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 2
flash_point_f
168.8 °F - closed cup
flash_point_c
76 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 366331-1G | 4061838097804 |
| 366331-5G | 4061838090430 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service





![2,5-Bis(trimethylstannyl)-thieno[3,2-b]thiophene 97%](/deepweb/assets/sigmaaldrich/product/structures/126/532/26557e94-858e-4c96-90de-ca88d84a8727/640/26557e94-858e-4c96-90de-ca88d84a8727.png)