343536
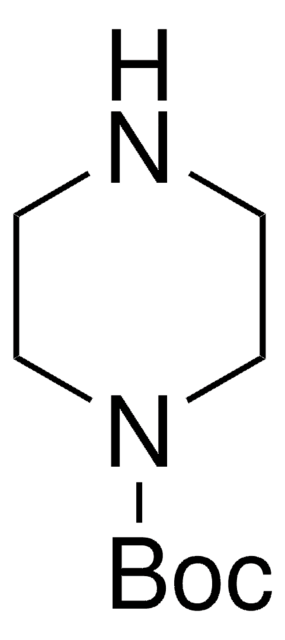
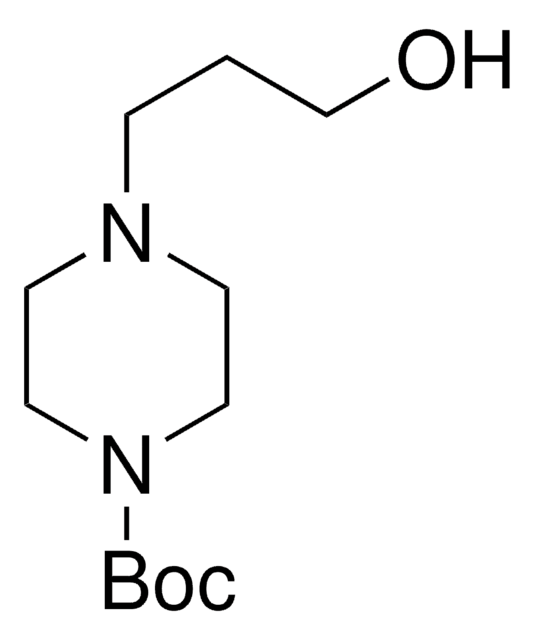
1-Boc-piperazine
97%
Synonym(s):
tert-Butyl piperazine-1-carboxylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H18N2O2
CAS Number:
Molecular Weight:
186.25
Beilstein/REAXYS Number:
879985
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
form
solid
mp
43-47 °C (lit.)
SMILES string
CC(C)(C)OC(=O)N1CCNCC1
InChI
1S/C9H18N2O2/c1-9(2,3)13-8(12)11-6-4-10-5-7-11/h10H,4-7H2,1-3H3
InChI key
CWXPZXBSDSIRCS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
1-Boc-piperazine is an N-Boc protected piperazine. Crosscoupling of 1-Boc-piperazine with aryl iodides using CuBr/1,1′-bi-2-naphthol (catalyst) and K3PO4 (base) has been reported. 1-Boc-piperazine undergoes Buchwald-Hartwig coupling reactions with aryl halides. 1-Boc-piperazine can be prepared in 80% yield via solvent-free N-Boc protection catalyzed by iodine.
Application
1-Boc-piperazine may be used in:
- preparation of series of (m-phenoxy)phenyl substituted piperazine derivatives
- termination step during synthesis of α,β-poly(2-oxazoline) lipopolymers via living cationic ring opening polymerization
- preparation of monosubstituted piperazines, e.g. in the synthesis of indazole DNA gyrase inhibitors
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
235.4 °F - closed cup
flash_point_c
113 °C - closed cup
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Florencio Zaragoza et al.
Journal of medicinal chemistry, 47(11), 2833-2838 (2004-05-14)
With the aim of identifying structurally novel, centrally acting histamine H(3) antagonists, arrays of monoacyldiamines were screened. This led to the discovery of a series of 1-alkyl-4-acylpiperazines which were potent antagonists at the human histamine H(3) receptor. The most potent
Dengfeng Dou et al.
Bioorganic & medicinal chemistry letters, 22(1), 377-379 (2011-11-29)
There is currently an unmet need for the development of small-molecule therapeutics for norovirus infection. The piperazine scaffold, a privileged structure embodied in many pharmacological agents, was used to synthesize an array of structurally-diverse derivatives which were screened for anti-norovius
Akihiko Tanitame et al.
Bioorganic & medicinal chemistry letters, 14(11), 2857-2862 (2004-05-06)
In this study, we report the design, synthesis and structure-activity relationships of novel indazole derivatives as DNA gyrase inhibitors with Gram-positive antibacterial activity. Our results show that selected compounds from this series exhibit potent antibacterial activity against Gram-positive bacteria including
α,ω-Functionalized Poly (2-Oxazoline) s Bearing Hydroxyl and Amino Functions.
Reif M and Jordan R.
Macromolecular Chemistry and Physics, 212(16), 1815-1824 (2011)
Ravi Varala et al.
The Journal of organic chemistry, 71(21), 8283-8286 (2006-10-10)
An efficient and practical protocol for the protection of various structurally and electronically divergent aryl and aliphatic amines using (Boc)2O in the presence of a catalytic amount of molecular iodine (10 mol %) under solvent-free conditions at ambient temperature is
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 343536-1G | 4061826758212 |
| 343536-25G | 4061826758229 |
| 343536-5G | 4061826758236 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service