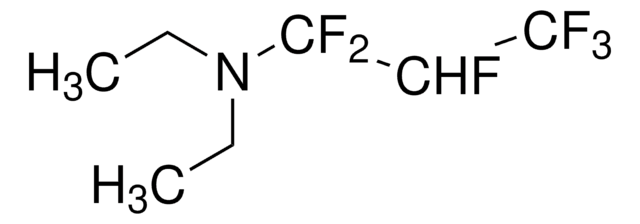
235253
(Diethylamino)sulfur trifluoride
95%
Synonym(s):
DAST, Diethylaminosulfur trifluoride
About This Item
Recommended Products
Quality Level
assay
95%
form
liquid
bp
30-32 °C/3 mmHg (lit.)
density
1.22 g/mL at 25 °C (lit.)
functional group
amine
storage temp.
2-8°C
SMILES string
CCN(CC)S(F)(F)F
InChI
1S/C4H10F3NS/c1-3-8(4-2)9(5,6)7/h3-4H2,1-2H3
InChI key
CSJLBAMHHLJAAS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Fluorinating agent: reaction with alcohols and carbonyl compounds, Review
- Review on nucleophilic fluorination.
- Catalyst for Friedel-Crafts allylation using tertiary cyclopropyl silyl ethers and the rearrangement of homoallylic alcohols to unsaturated aldehydes.
- Early introduction of a fluoromethyl group stabilizes the epoxide during further manipulations in the synthesis of 26-fluoro-epothilone.
- Fluorinating agent for a variety of compounds, including thioethers, alkenols, and cyanohydrins.
- Reagent for gem difluorination of ketopipecolinic acids.
Replaced by
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Self-react. D - Skin Corr. 1A
supp_hazards
Storage Class
5.2 - Organic peroxides and self-reacting hazardous materials
wgk_germany
WGK 3
flash_point_f
73.4 °F
flash_point_c
23 °C
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 235253-1G | 4061838784971 |
| 235253-5G | 4061838784995 |
| 235253-125G | 4061838231222 |
| 235253-250G | |
| 235253-25G | 4061838784988 |
| 235253-4X25G |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)