226890
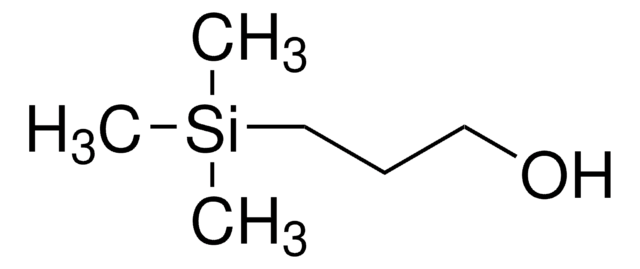
2-(Trimethylsilyl)ethanol
96%
Synonym(s):
(2-Hydroxyethyl)trimethylsilane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
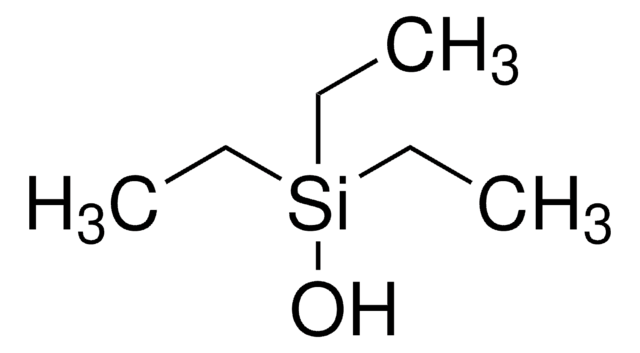
(CH3)3SiCH2CH2OH
CAS Number:
Molecular Weight:
118.25
Beilstein/REAXYS Number:
1732034
EC Number:
MDL number:
UNSPSC Code:
12352000
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
96%
form
liquid
refractive index
n20/D 1.423 (lit.)
bp
71-73 °C/35 mmHg (lit.)
density
0.825 g/mL at 25 °C (lit.)
SMILES string
C[Si](C)(C)CCO
InChI
1S/C5H14OSi/c1-7(2,3)5-4-6/h6H,4-5H2,1-3H3
InChI key
ZNGINKJHQQQORD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Protecting reagent for carboxyl and phosphate groups.
Used to synthesize Teoc-protected amines via alcoholysis of the corresponding isocyanates.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron Letters, 33, 7685-7685 (1992)
Tetrahedron Letters, 35, 757-757 (1994)
Seth L Crawley et al.
Organic letters, 8(18), 3995-3998 (2006-08-25)
A new protocol for generating aza-ortho-xylylenes via acid-catalyzed or fluoride-promoted ring opening of 2-(2-acylaminophenyl)aziridines is described. This methodology has been exploited in the rapid construction of a hexacyclic substructure of communesin B.
A Aberman et al.
Biochimica et biophysica acta, 791(2), 278-280 (1984-12-07)
Several trimethylsilyl derivatives were found to be ligands of acetylcholinesterase (acetylcholine acetylhydrolase, EC 3.1.1.7): trimethylsilylethyl acetate (III) and trimethylsilylmethyl acetate (V) are substrates of the enzyme, whereas trimethylsilylethanol (VIII) is a competitive inhibitor. The silicon compounds have kinetic parameters similar
Journal of the Chemical Society. Perkin Transactions 1, 2639-2639 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service