225649
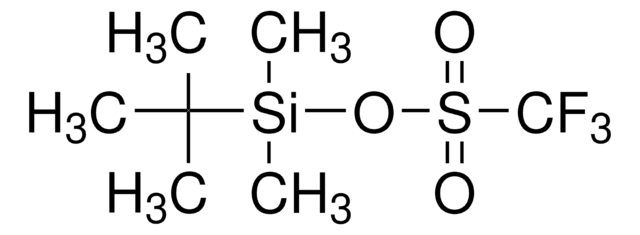
Trimethylsilyl trifluoromethanesulfonate
99%
Synonym(s):
TMS triflate, TMSOTf, Trifluoromethanesulfonic acid trimethylsilylester
About This Item
Recommended Products
Quality Level
assay
99%
form
liquid
refractive index
n20/D 1.36 (lit.)
bp
77 °C/80 mmHg (lit.)
density
1.228 g/mL at 25 °C (lit.)
SMILES string
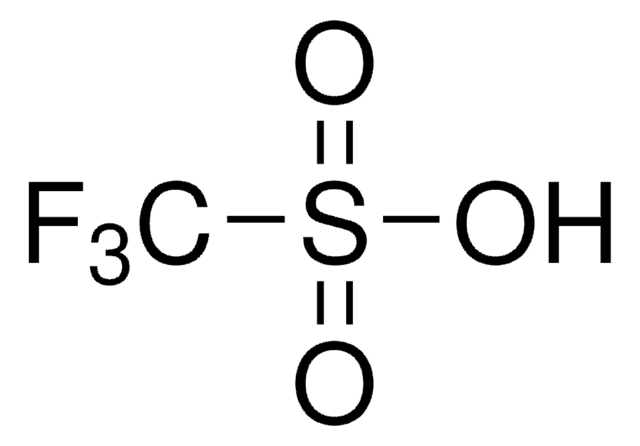
C[Si](C)(C)OS(=O)(=O)C(F)(F)F
InChI
1S/C4H9F3O3SSi/c1-12(2,3)10-11(8,9)4(5,6)7/h1-3H3
InChI key
FTVLMFQEYACZNP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
It can be used:
- As a silylating agent for the synthesis of trimethylsilyl-enol ethers from esters of α-diazoacetoacetic acid.
- To activate benzyl and allyl ethers for the alkylation of sulfides.
- To facilitate the conversion of Diels-Alder adducts of Danishefsky′s diene to cyclohexenones without the formation of methoxy ketone by-product.
- To prepare difluoroboron triflate etherate, a powerful Lewis acid especially in acetonitrile solvent.
- As a reagent in a Dieckmann-like cyclization of ester-imides and diesters.
It may also be used to catalyze:
- Acylation of alcohols with acid anhydrides.
- Reductive coupling of carbonyl compounds with trialkylsilanes to form symmetrical ethers.
- Glycosidation of 4-demethoxydaunomycinones with 1-O-acyl-L-daunosamine derivatives.
accessory
signalword
Danger
hcodes
Hazard Classifications
Flam. Liq. 3 - Skin Corr. 1B
supp_hazards
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
77.0 °F - closed cup
flash_point_c
25 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service