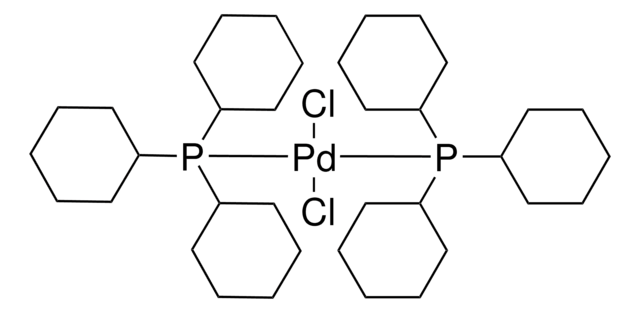
208671
Bis(triphenylphosphine)palladium(II) dichloride
98%
Synonym(s):
Dichlorobis(triphenylphosphine)palladium(II), Palladium(II)bis(triphenylphosphine) dichloride, PdCl2(PPh3)2
About This Item
Recommended Products
Quality Level
assay
98%
form
solid
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
SMILES string
Cl[Pd]Cl.c1(P(c2ccccc2)c3ccccc3)ccccc1.c4(P(c5ccccc5)c6ccccc6)ccccc4
InChI
1S/2C18H15P.2ClH.Pd/c2*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;;/h2*1-15H;2*1H;/q;;;;+2/p-2
InChI key
YNHIGQDRGKUECZ-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Coupling of 2-iodoanisole and terminal alkynes to synthesize 2,3-disubstituted benzofurans.
- Copper-free Sonogashira cross-coupling reaction to synthesize diphenylacetylene.
- Regioselective hydrocarboxylation of styrene.
- Negishi coupling of fluoroarylzinc pivalates to prepare fluorinated oligophenyls.
- Coupling of iodo-α-β-unsaturated esters to afford tetrasubstituted olefins.
Related product
signalword
Warning
hcodes
Hazard Classifications
Aquatic Chronic 4 - Skin Sens. 1A
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
A variety of transition-metal catalysts for the Suzuki coupling reaction are now available in our catalog. The majority of these catalysts are palladium- and nickelbased, typically utilizing phosphine-derived ligands.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)