推荐产品
等級
pharmaceutical primary standard
API 家族
doxorubicin
製造商/商標名
USP
mp
216 °C (dec.) (lit.)
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
−20°C
SMILES 字串
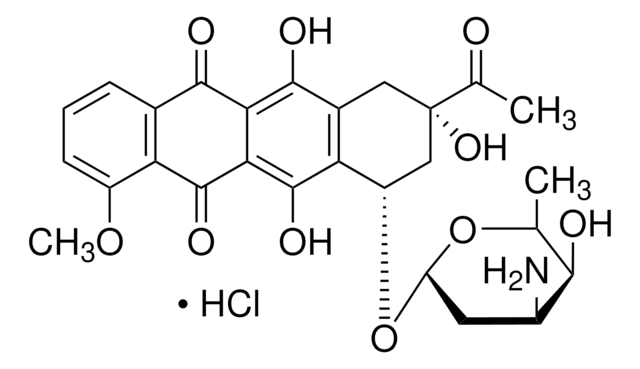
Cl[H].COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](N)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO
InChI
1S/C27H29NO11.ClH/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34;/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3;1H/t10-,13-,15-,17-,22+,27-;/m0./s1
InChI 密鑰
MWWSFMDVAYGXBV-RUELKSSGSA-N
基因資訊
human ... TOP2A(7153)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Doxorubicin hydrochloride USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Doxorubicin Hydrochloride Injection
- Doxorubicin Hydrochloride for Injection
- Epirubicin Hydrochloride
- Epirubicin Hydrochloride Injection
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Carc. 1B - Muta. 1B - Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Doxorubicin Hydrochloride Injection
United States Pharmacopeia, 42(4), 1514-1514 (2022)
Xin Ma et al.
Proceedings of the National Academy of Sciences of the United States of America, 111(17), 6389-6394 (2014-04-16)
A critical challenge for chemotherapy is the development of chemoresistance in breast cancer. However, the underlying mechanisms and validated predictors remain unclear. Extracellular vesicles (EVs) have gained attention as potential means for cancer cells to share intracellular contents. In adriamycin-resistant
C Main et al.
Health technology assessment (Winchester, England), 10(9), 1-132 (2006-03-21)
To examine the clinical effectiveness and cost-effectiveness of intravenous formulations of topotecan monotherapy, pegylated liposomal doxorubicin hydorocholoride (PLDH) monotherapy and paclitaxel used alone or in combination with a platinum-based compound for the second-line or subsequent treatment of advanced ovarian cancer.
Shuai Li et al.
Journal of biomedical nanotechnology, 10(8), 1480-1489 (2014-07-16)
Amphiphilic copolymers have been paid much attention for controlled drug release for many years due to their obvious advantages. In this study, an acid-triggered drug carrier system capable of rapid intracellular drug release is investigated for potential tumor therapy. The
Angela A Salim et al.
Organic letters, 16(19), 5036-5039 (2014-09-23)
During a search for inhibitors of oncogenic K-Ras, we detected two known and two new examples of the rare neoantimycin structure class from a liquid cultivation of Streptomyces orinoci, and reassigned/assigned structures to all based on detailed spectroscopic analysis and
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门