G6137
Glutathione Peroxidase from bovine erythrocytes
lyophilized powder, ≥300 units/mg protein
Synonym(s):
GSH-Px, Glutathione:hydrogen-peroxide oxido-reductase
About This Item
Recommended Products
biological source
bovine erythrocytes
Quality Level
form
lyophilized powder
specific activity
≥300 units/mg protein
mol wt
84.5 kDa
composition
Protein, 10-30% modified Warburg-Christian
shipped in
dry ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
Application
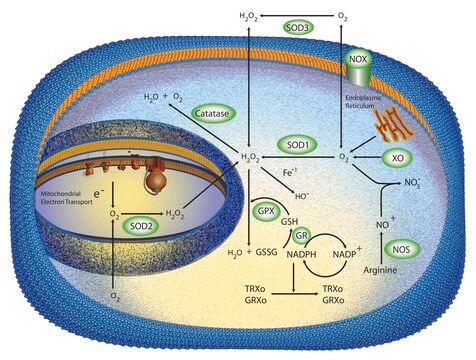
Biochem/physiol Actions
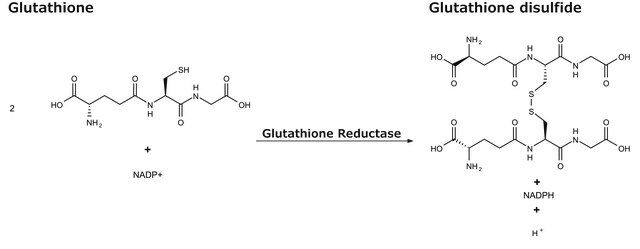
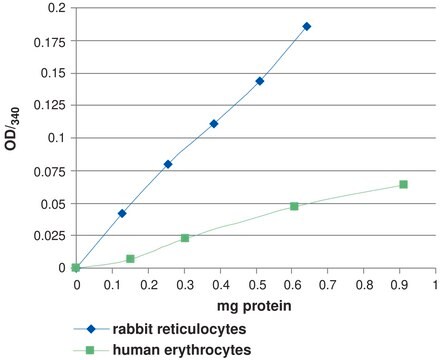
Unit Definition
Physical form
Other Notes
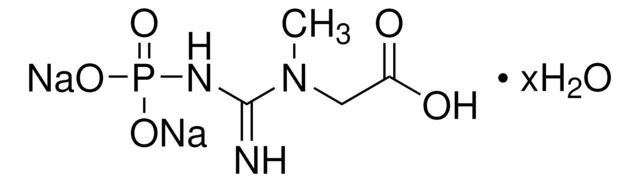
Related product
signalword
Danger
hcodes
pcodes
Hazard Classifications
Resp. Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Oxidative stress is mediated, in part, by reactive oxygen species produced by multiple cellular processes and controlled by cellular antioxidant mechanisms such as enzymatic scavengers or antioxidant modulators. Free radicals, such as reactive oxygen species, cause cellular damage via cellular.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service