Kluczowe dokumenty
T0575
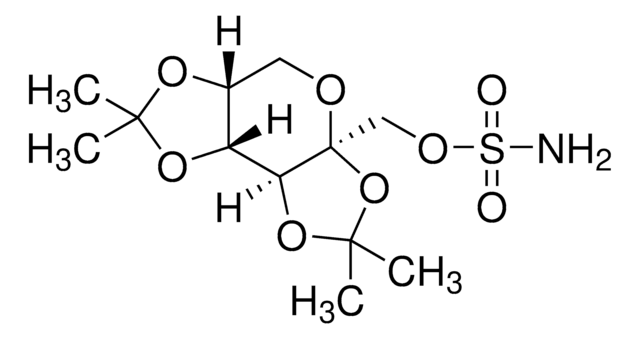
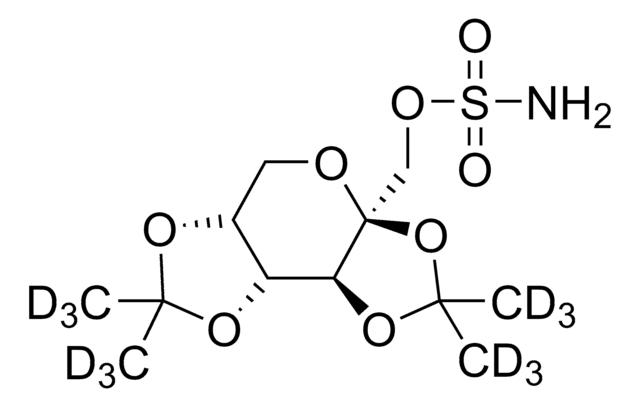
Topiramate
≥98% (HPLC), solid
Synonim(y):
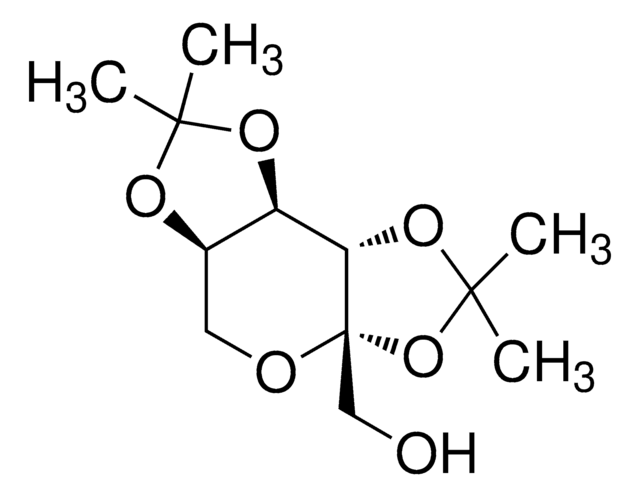
2,3:4,5-Bis-O-(1-methylethylidene)-36-D-fructo-pyranose sulfamate, McN 4853, RWJ 17021, Topamax
About This Item
Polecane produkty
Poziom jakości
Próba
≥98% (HPLC)
Formularz
solid
kolor
white
rozpuszczalność
DMSO: 40 mg/mL
inicjator
Johnson & Johnson
temp. przechowywania
2-8°C
ciąg SMILES
NS(OC[C@]12[C@](OC(C)(C)O2)([H])[C@@]3([H])[C@@](OC(C)(C)O3)([H])CO1)(=O)=O
InChI
1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1
Klucz InChI
KJADKKWYZYXHBB-XBWDGYHZSA-N
informacje o genach
human ... CA1(759) , CA2(760) , CA4(762) , CA5A(763) , CA5B(11238) , CA9(768) , GABRA1(2554) , GABRA2(2555) , GABRA3(2556) , GABRA4(2557) , GABRA5(2558) , GABRA6(2559) , GABRB1(2560) , GABRB2(2561) , GABRB3(2562) , GABRD(2563) , GABRE(2564) , GABRG1(2565) , GABRG2(2566) , GABRG3(2567) , GABRP(2568) , GABRQ(55879) , GRIA1(2890) , GRIA2(2891) , GRIA3(2892) , GRIA4(2893) , GRIK1(2897) , GRIK2(2898) , GRIK3(2899) , GRIK4(2900) , GRIK5(2901) , SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
mouse ... Car5a(12352)
rat ... Car2(54231) , Car4(29242)
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
- to study the antiaggressive effects of topiramate in mice.
- to study of the effect of topiramate in promoting neurite outgrowth post nerve injury in fetal rat cortical and hippocampal tissues.
- as a mitochondrial carbonic anhydrase inhibitor to block the effects of high glucose or glucotoxicity.
- to reduce cell death and mitochondrial dysfunction induced by the administration of kainic acid.
Działania biochem./fizjol.
Cechy i korzyści
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organy docelowe
Respiratory system
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej