SRP9000
Prolactin human
human, recombinant, expressed in HEK 293 cells
Synonim(y):
PRL
About This Item
Polecane produkty
pochodzenie biologiczne
human
Poziom jakości
rekombinowane
expressed in HEK 293 cells
sterylność
non-sterile
Próba
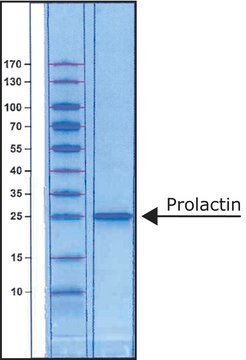
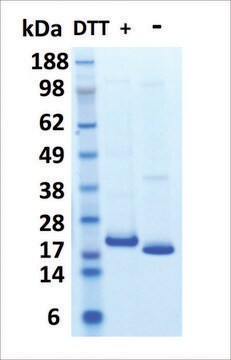
≥95% (SDS-PAGE)
Postać
liquid
siła działania
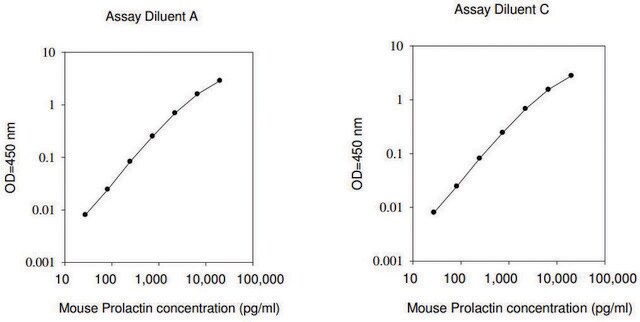
≤2 ng/mL Nb2-11 cells proliferation EC50
okres trwałości
2 yr
masa cząsteczkowa
23 kDa
metody
cell culture | mammalian: suitable
zanieczyszczenia
≤1 EU/μg protein Endotoxin level
temp. przechowywania
−20°C
informacje o genach
human ... prl(5617)
Opis ogólny
Prolactin (PRL) is a multifunctional polypeptide hormone primarily produced by the lactotrophic cells of the anterior pituitary gland in vertebrates.
Zastosowanie
- in in vitro experiments to examine its effects in sleep-like concentrations on T-cell migration
- to study its effects on claudin 2 (CLDN2) expression in the Caco-2 intestinal epithelial cell model
- in microplate assays to demonstrate the specificity of the antibodies for vasoinhibin
Działania biochem./fizjol.
Postać fizyczna
Uwaga dotycząca przygotowania
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Repr. 1B
Kod klasy składowania
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej