ML0015
Aspartate Metabolite Library
Synonim(y):
Biblioteka metabolitów kwasu asparaginowego
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Kod UNSPSC:
12352209
NACRES:
NA.25
Polecane produkty
opis
Aspartate pathway
Poziom jakości
Formularz
solid
Zastosowanie
metabolomics
temp. przechowywania
−20°C
Opis ogólny
Kwas asparaginowy (lub asparaginian) jest aminokwasem nieistotnym, co oznacza, że jest naturalnie syntetyzowany przez ssaki. Asparaginian pełni wiele ról biochemicznych:
W konformacji L kwas asparaginowy jest budulcem w produkcji białek, a także pomaga w wielu funkcjach organizmu, w tym w cyklu mocznikowym, glukoneogenezie i cyklu Krebsa, procesie generującym adenozynotrifosforan (ATP). Kwas asparaginowy działa również jako neuroprzekaźnik. Konformacja D-asparaginianu jest powiązana z neurogenezą i układami hormonalnymi.
W konformacji L kwas asparaginowy jest budulcem w produkcji białek, a także pomaga w wielu funkcjach organizmu, w tym w cyklu mocznikowym, glukoneogenezie i cyklu Krebsa, procesie generującym adenozynotrifosforan (ATP). Kwas asparaginowy działa również jako neuroprzekaźnik. Konformacja D-asparaginianu jest powiązana z neurogenezą i układami hormonalnymi.
Zastosowanie
Biblioteka metabolitów asparaginianu to zestaw zawierający wybór 23 metabolitów biorących udział w metabolizmie asparaginianu, które mogą być wykorzystywane do ogólnych badań, jako odczynniki lub jako związki referencyjne w procedurach analitycznych.
Działania biochem./fizjol.
Rola i metabolity asparaginianu:
- Asparaginian jest syntetyzowany przez transaminację szczawiooctanu poprzez działanie aminotransferazy asparaginianowej i 5′-fosforanu pirydoksalu. Syntaza aspartylo-tRNA może następnie połączyć asparaginian z aspartylo-tRNA w celu syntezy białek.
- Asparaginian przenosi równoważniki redukujące w mitochondrialnym czółenku jabłczanowo-asparaginianowym, które wykorzystuje gotową interkonwersję asparaginianu i szczawiooctanu.
- SyntazaN-acetyloasparaginianu, obecna w cytoplazmie, przekształca asparaginian w N-acetyloasparaginian, metabolit mózgu, który reguluje dopaminę.
- Asparagina jest biosyntetyzowana przez syntetazę asparaginy z asparaginianu, glutaminy i ATP. Asparagina bierze udział w metabolicznej kontroli funkcji komórek w tkance nerwowej i mózgowej.
- Kwas argininobursztynowy jest syntetyzowany z asparaginianu, cytruliny i ATP poprzez działanie syntetazy argininobursztynianu, jednego z enzymów cyklu mocznikowego. W tym szlaku metabolicznym neurotoksyczny amoniak, wytwarzany przez katabolizm białek, jest przekształcany w mocznik w wątrobie.
- Kwas fumarowy jest syntetyzowany z kwasu argininobursztynowego za pośrednictwem liazy argininobursztynianowej, która jest enzymem w cyklu kwasu cytrynowego.
- Kwas inozynowy, kwas asparaginowy i GTP są przekształcane do GDP i AMP przez izozym 1 syntetazy adenylobursztynianu. Proces ten jest zaangażowany w cykl nukleotydów purynowych, który reguluje poziom nukleotydów w różnych tkankach.
- Transkarbamoilaza asparaginianowa katalizuje syntezę N-karbamoilo-L-asparaginianu z fosforanu karbamoilu i asparaginianu, które biorą udział w biosyntezie pirymidyn de novo.
- Beta alanina powstaje w wyniku dekarboksylacji asparaginianu przez dekarboksylazę glutaminianową 1 w cytoplazmie.
- L-asparaginian jest przekształcany w D-asparaginian poprzez działanie racemazy D-asparaginianu. D-asparaginian przyczynia się do syntezy i uwalniania glukokortykoidów, prolaktyny, oksytocyny i steroidów. D-asparaginian odgrywa ważną rolę w aktywności mózgu ssaków.
Komponenty
Contains 10 mg each of Aspartate metabolism metabolite standards packaged individually.
Ta strona może zawierać tekst przetłumaczony maszynowo.
Elementy zestawu są też dostępne oddzielnie
Numer produktu
Opis
Karta charakterystyki
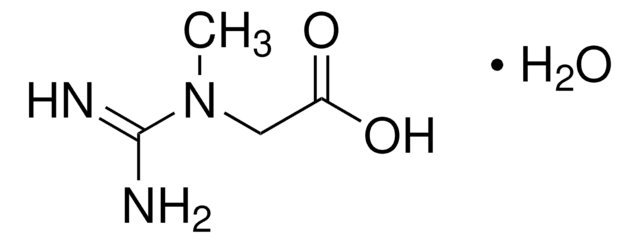
- A2252Adenosine 5′-monophosphate monohydrate, from yeast, ≥97%Karta charakterystyki
- A2383Adenosine 5′-triphosphate disodium salt hydrate, Grade I, ≥99%, from microbialKarta charakterystyki
- A5707Argininosuccinic acid disodium salt hydrate, ≥80%Karta charakterystyki
- 146064β-Alanine, 99%Karta charakterystyki
- C4135Carbamyl phosphate disodium salt, ≥80%Karta charakterystyki
- C7629L-Citrulline, ≥98% (TLC)Karta charakterystyki
- 219096D-Aspartic acid, ReagentPlus®, 99%Karta charakterystyki
- F6625Flavin adenine dinucleotide disodium salt hydrate, ≥95% (HPLC), powderKarta charakterystyki
- 47910Fumaric acid, ≥99.0% (T)Karta charakterystyki
- G7252Guanosine 5′-diphosphate tris salt from Saccharomyces cerevisiae, Type VI, ≥92.5%Karta charakterystyki
- G9002Guanosine 5′-triphosphate tris salt, ≥93% (HPLC), powderKarta charakterystyki
- I2879Inosine 5′-monophosphate from Saccharomyces cerevisiae, ≥98%Karta charakterystyki
- A5006L-Arginine, reagent grade, ≥98%Karta charakterystyki
- A0884L-Asparagine, ≥98% (HPLC)Karta charakterystyki
- 11189L-Aspartic acid, BioUltra, ≥99.5% (T)Karta charakterystyki
- G1251L-Glutamic acid, ReagentPlus®, ≥99% (HPLC)Karta charakterystyki
- G3126L-Glutamine, ReagentPlus®, ≥99% (HPLC)Karta charakterystyki
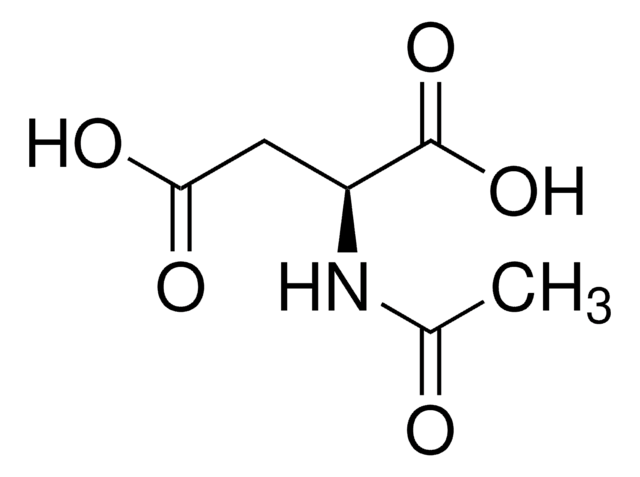
- 00920N-Acetyl-L-aspartic acid, ≥99.0% (T)Karta charakterystyki
- O4126Oxaloacetic acid, ≥97% (HPLC)Karta charakterystyki
- K1750α-Ketoglutaric acid, ≥98.5% (NaOH, titration)Karta charakterystyki
- P9255Pyridoxal 5′-phosphate hydrate, ≥98%Karta charakterystyki
- P8010Sodium pyrophosphate tetrabasic, ≥95%Karta charakterystyki
- 69037Ureidosuccinic acid, 98.0-102.0% (T)Karta charakterystyki
Zobacz wszystko (23)
produkt powiązany
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Oral - Eye Dam. 1
Kod klasy składowania
11 - Combustible Solids
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumenty section.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Pyrimidine Biosynthesis
Lennarz W J, et al.
Encyclopedia of Biological Chemistry, 600-605 (2004)
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej