Wszystkie zdjęcia(3)
Kluczowe dokumenty
D9761
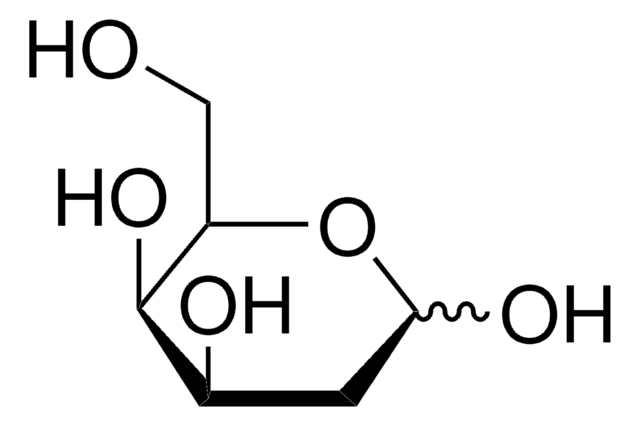
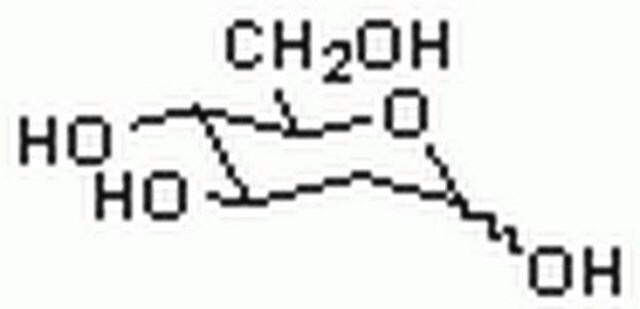
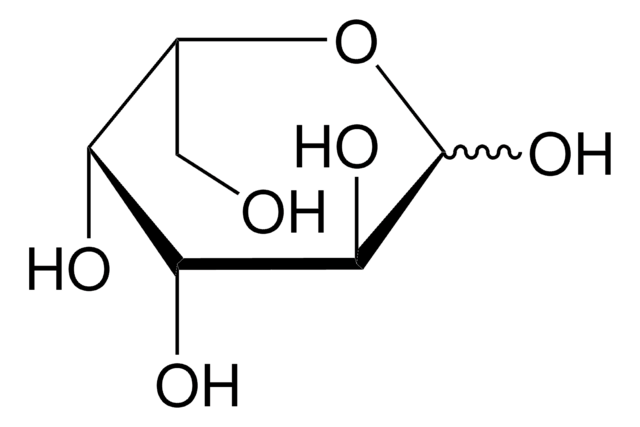
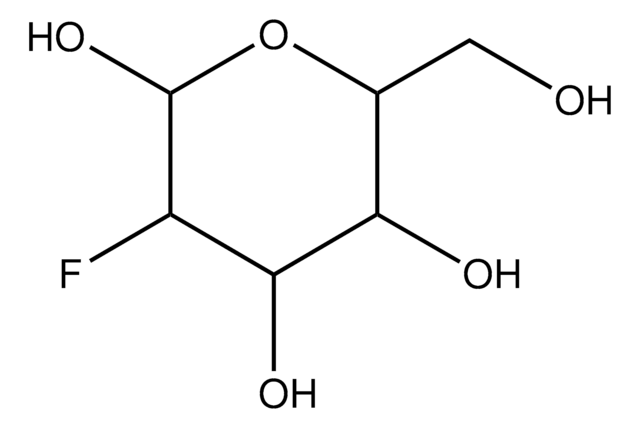
6-Deoxy-D-glucose
Synonim(y):
D-Isorhamnose, Epifucose, Quinovose
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
C6H12O5
Numer CAS:
Masa cząsteczkowa:
164.16
Beilstein:
1723317
Numer WE:
Numer MDL:
Kod UNSPSC:
12352200
Identyfikator substancji w PubChem:
NACRES:
NA.52
Polecane produkty
klasa czystości
Molecular Biology
for molecular biology
Poziom jakości
Próba
≥98% (TLC)
Formularz
powder
temp. przechowywania
−20°C
ciąg SMILES
C[C@H]1OC(O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C6H12O5/c1-2-3(7)4(8)5(9)6(10)11-2/h2-10H,1H3/t2-,3-,4+,5-,6?/m1/s1
Klucz InChI
SHZGCJCMOBCMKK-GASJEMHNSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
6-Deoxy-D-glucose is a structural homolog of D-glucose (dextrose) and stable analog. It lacks the hydroxyl group at carbon 6 position. It is also an analog of mannose.
Zastosowanie
6-Deoxy-D-glucose has been used as a standard in the circular dichroism measurements. It has also been used as sugar to incubate starved Dictyostelium HMX44A.atg1-1 cells for microscopy studies.
Działania biochem./fizjol.
2-Deoxy-D-glucose (2-DG) is used as a glycolytic inhibitor in studying the biological function of glucose. It is not metabolized, induces endoplasmic reticulum stress and hence, blocks the carbohydrate metabolism in cancer cells. It has therapeutic potential in targeting chemo-resistant hypoxic cancer cells. 2-DG halts the N-linked glycosylation by replacing mannose.
Ta strona może zawierać tekst przetłumaczony maszynowo.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
D Granot et al.
Proceedings of the National Academy of Sciences of the United States of America, 88(13), 5724-5728 (1991-07-01)
Nutrients play a critical role in the decision to initiate a new cell cycle. Addition of nutrients to arrested cells such as stationary-phase cells and spores induces them to begin growth. We have analyzed the nutrients required to induce early
A H Romano
Journal of bacteriology, 152(3), 1295-1297 (1982-12-01)
6-Deoxy-D-glucose, a structural homomorph of D-glucose which lacks a hydroxyl group at carbon 6 and thus cannot be phosphorylated, is transported by Saccharomyces cerevisiae via a facilitated diffusion system with affinity equivalent to that shown with D-glucose. This finding supports
C Laporte et al.
Cell death and differentiation, 14(2), 266-274 (2006-07-01)
While necrotic cell death is attracting considerable interest, its molecular bases are still poorly understood. Investigations in simple biological models, taken for instance outside the animal kingdom, may benefit from less interference from other cell death mechanisms and from better
Cristina De Castro et al.
Glycobiology, 23(3), 346-353 (2012-10-19)
A major virulence factor for Yersinia pseudotuberculosis is lipopolysaccharide, including O-polysaccharide (OPS). Currently, the OPS based serotyping scheme for Y. pseudotuberculosis includes 21 known O-serotypes, with genetic and structural data available for 17 of them. The completion of the OPS
A J Fry et al.
Molecular and biochemical parasitology, 60(1), 9-18 (1993-07-01)
Kinetic parameters for entry of D-fructose into Trypanosoma brucei brucei have been determined. The net uptake of D-fructose was found to be rapid and occurred at a rate which was comparable with that observed for uptake of D-glucose. The Km
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej