Key Documents
D4641
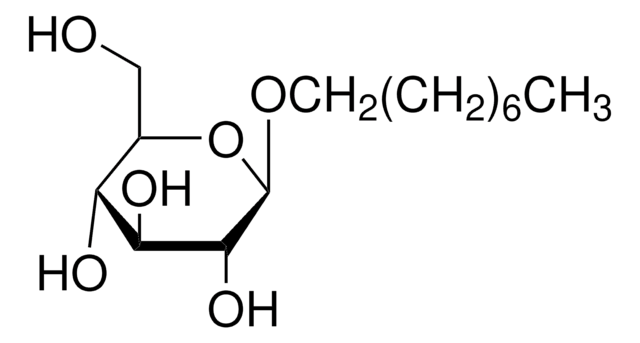
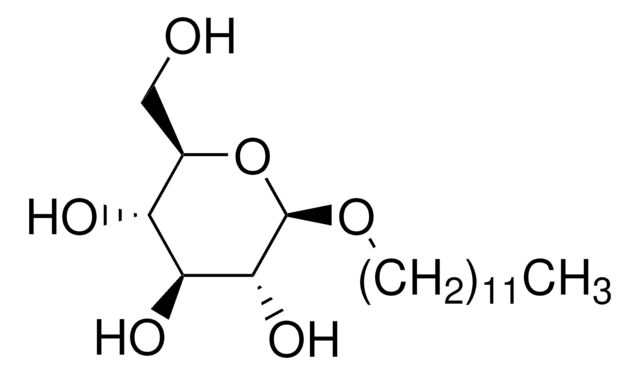
n-Dodecyl β-D-maltoside
≥98% (GC)
Synonim(y):
DDM, Lauryl-β-D-maltoside
About This Item
Polecane produkty
pochodzenie biologiczne
corn
Poziom jakości
opis
non-ionic
Próba
≥98% (GC)
Postać
powder
masa cząsteczkowa
micellar avg mol wt 50,000
liczba agregacji
98
metody
protein quantification: suitable
zanieczyszczenia
<1.5% water (Karl Fischer)
CMC
0.15 mM (20-25°C)
mp
224-226 °C (lit.)
rozpuszczalność
water: 50 mg/mL, clear to very slightly hazy, colorless
temp. przechowywania
−20°C
ciąg SMILES
CCCCCCCCCCCCO[C@@H]1O[C@H](CO)[C@@H](O[C@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O
InChI
1S/C24H46O11/c1-2-3-4-5-6-7-8-9-10-11-12-32-23-21(31)19(29)22(16(14-26)34-23)35-24-20(30)18(28)17(27)15(13-25)33-24/h15-31H,2-14H2,1H3/t15-,16-,17-,18+,19-,20-,21-,22-,23-,24-/m1/s1
Klucz InChI
NLEBIOOXCVAHBD-QKMCSOCLSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
In addition to its primary function, N-dodecyl-β-D-maltoside has diverse applications, including the purification and stabilization of RNA polymerase, the detection of protein-lipid interactions, and serving as a substrate for glucosyl and xylosyl transfer by glycogenin. Its mild and non-denaturing properties have further led to its utilization in protein-anesthetic studies. Overall, N-dodecyl-β-D-maltoside emerges as a versatile detergent with a broad range of applications in membrane protein research and beyond. Its pivotal role in preserving protein structure and function establishes it as an indispensable tool for exploring the intricacies of membrane biology.
Zastosowanie
Cechy i korzyści
- Highly versatile surfactant for your cell biology and biochemical research
- Suitable for protein quantification
Inne uwagi
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Protokoły
This page shows how to solubilize membrane proteins with products from GE Healthcare.
Na tej stronie przedstawiono sposób solubilizacji białek błonowych za pomocą produktów firmy Cytiva.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej