Kluczowe dokumenty
D141
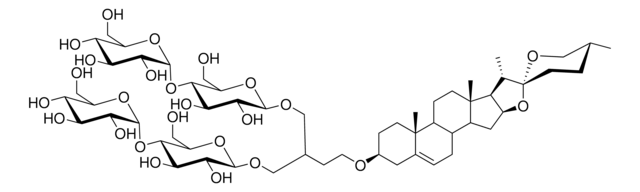
Digitonin
Used as non-ionic detergent
Synonim(y):
Digitin
About This Item
Polecane produkty
pochodzenie biologiczne
plant seeds (Digitalis purpurea)
Formularz
powder
aktywność optyczna
[α]20/D −54°, c = 2.8 in methanol(lit.)
masa cząsteczkowa
micellar avg mol wt 70,000
liczba agregacji
60
zanieczyszczenia
<6.0% water
CMC
<0.5 mM (20-25°C)
<0.5 mM (20-25°C)
mp
230-240 °C (dec.) (lit.)
rozpuszczalność
H2O: ~5 % (w/v) (solubilized by heating to 95 °C - 98 °C and then cooling to room temp.)
ciąg SMILES
C[C@@]12[C@]([C@@H]3C)([H])[C@](O[C@]34CC[C@@H](C)CO4)([H])[C@@H](O)[C@@]1([H])[C@@](CC[C@]5([H])[C@@]6(C[C@@H](O)[C@H](O[C@]([C@@H]([C@@H](O)[C@H]7O[C@@](O[C@H](CO)[C@@H](O)[C@@H]8O[C@@](OC[C@@H](O)[C@@H]9O)([H])[C@@H]9O)([H])[C@@H]8O[C@@](O[C@H](CO)[C@H
InChI
1S/C56H92O29/c1-19-7-10-56(75-17-19)20(2)31-45(85-56)37(67)32-22-6-5-21-11-26(24(61)12-55(21,4)23(22)8-9-54(31,32)3)76-50-42(72)39(69)44(30(16-60)80-50)81-53-48(47(36(66)29(15-59)79-53)83-49-40(70)33(63)25(62)18-74-49)84-52-43(73)46(35(65)28(14-58)78-52)82-51-41(71)38(68)34(64)27(13-57)77-51/h19-53,57-73H,5-18H2,1-4H3/t19-,20+,21+,22-,23+,24-,25-,26-,27-,28-,29-,30-,31+,32-,33+,34-,35+,36-,37+,38+,39-,40-,41-,42-,43-,44+,45-,46+,47+,48-,49+,50-,51+,52+,53+,54-,55+,56-/m1/s1
Klucz InChI
UVYVLBIGDKGWPX-KUAJCENISA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Opis ogólny
Moreover, Digitonin selectively permeabilizes the cholesterol-rich plasma membrane, leaving organelle membranes intact. This feature facilitates the extraction and purification of specific organelles, contributing to the study of their functions and roles in cellular processes. The detergent′s capability to permeabilize diverse cell types allows the study of intracellular components and processes by introducing molecules, antibodies, or enzymes into cells. Additionally, Digitonin finds application in immunocytochemistry experiments, enabling the labeling and detection of intracellular proteins and structures.
Zastosowanie
Działania biochem./fizjol.
Cechy i korzyści
Jakość
Inne uwagi
produkt podobny
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 3 Oral - STOT RE 2
Kod klasy składowania
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Protokoły
Na tej stronie przedstawiono sposób solubilizacji białek błonowych za pomocą produktów firmy Cytiva.
This page shows how to solubilize membrane proteins with products from GE Healthcare.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej