C4879
α-Chymotrypsinogen A from bovine pancreas
essentially salt-free, lyophilized powder
Synonim(y):
zymogen chymotrypsyna A
About This Item
Polecane produkty
pochodzenie biologiczne
bovine pancreas
Poziom jakości
typ
Type II
Formularz
essentially salt-free, lyophilized powder
aktywność właściwa
≥40 units/mg solid
masa cząsteczkowa
25,656 Da by calculation
oczyszczone przez
6× crystallization
rozpuszczalność
1 mM HCl: soluble 10 mg/mL, clear, colorless
numer dostępu UniProt
obecność zanieczyszczeń
α-chymotrypsin ≤1 U/mg (prior to activation by trypsin)
temp. przechowywania
−20°C
informacje o genach
cow ... CTRB1(618826)
Powiązane kategorie
Opis ogólny
Zastosowanie
Działania biochem./fizjol.
Definicja jednostki
Inne uwagi
Zastosowanie
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Separation of Ribonuclease A from bovine pancreas, Type I-A, powder, ≥60% RNase A basis (SDS-PAGE), ≥50 Kunitz units/mg protein; α-Chymotrypsinogen A from bovine pancreas, essentially salt-free, lyophilized powder; Cytochrome c from bovine heart, ≥95% based on Mol. Wt. 12,327 basis; Lysozyme from chicken egg white, lyophilized powder, protein ≥90 %, ≥40,000 units/mg protein
Separation of Ribonuclease A from bovine pancreas, Type I-A, powder, ≥60% RNase A basis (SDS-PAGE), ≥50 Kunitz units/mg protein; α-Chymotrypsinogen A from bovine pancreas, essentially salt-free, lyophilized powder; Cytochrome c from bovine heart, ≥95% based on Mol. Wt. 12,327 basis; Lysozyme from chicken egg white, lyophilized powder, protein ≥90 %, ≥40,000 units/mg protein
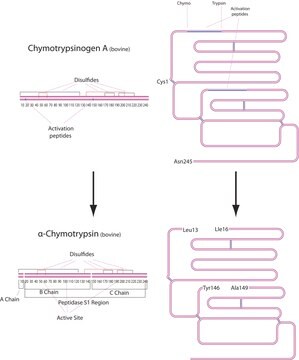
Analytical Enzyme Chymotrypsin: Chymotrypsin is produced in the acinar cells of the pancreas as the inactive precursor, chymotrypsinogen.
Enzym analityczny Chymotrypsyna: Chymotrypsyna jest wytwarzana w komórkach gruczołowych trzustki jako nieaktywny prekursor, chymotrypsynogen.
Chromatograms
application for HPLCNasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej