Kluczowe dokumenty
A4487
Aphidicolin, Ready Made Solution
from Nigrospora sphaerica
Synonim(y):
ICI 69653, NSC-234714
About This Item
Polecane produkty
pochodzenie biologiczne
Nigrospora sphaerica
Poziom jakości
ciśnienie pary
0.55 hPa ( 20 °C)
Formularz
liquid
kolor
clear colorless
rozpuszczalność
H2O: miscible (completely)
spektrum działania antybiotyku
neoplastics
viruses
Tryb działania
DNA synthesis | interferes
enzyme | inhibits
temp. przechowywania
−20°C
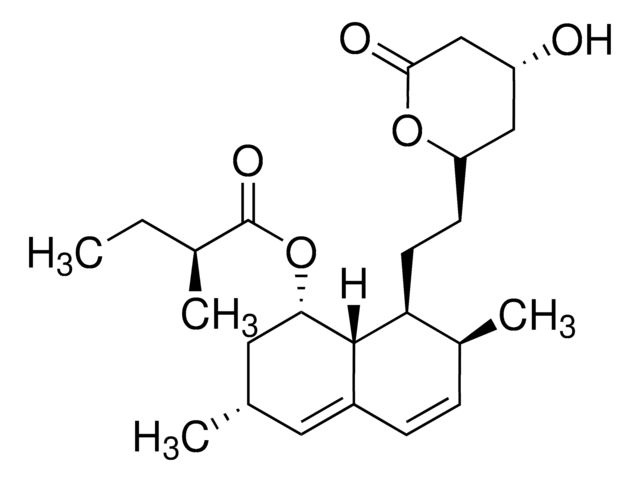
ciąg SMILES
O[C@]1([C@H]2C[C@@]3([C@@]4([C@H]([C@@]([C@@H](CC4)O)(CO)C)CC[C@H]3C2)C)CC1)CO
InChI
1S/C20H34O4/c1-17(11-21)15-4-3-13-9-14-10-19(13,7-8-20(14,24)12-22)18(15,2)6-5-16(17)23/h13-16,21-24H,3-12H2,1-2H3/t13-,14+,15-,16+,17-,18-,19-,20-/m0/s1
Klucz InChI
NOFOAYPPHIUXJR-APNQCZIXSA-N
Opis ogólny
Zastosowanie
Działania biochem./fizjol.
Opakowanie
Postać fizyczna
Inne uwagi
Kod klasy składowania
10 - Combustible liquids
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
188.6 °F - closed cup
Temperatura zapłonu (°C)
87 °C - closed cup
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej