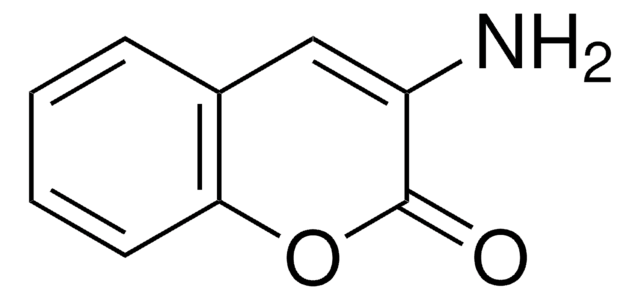
28605
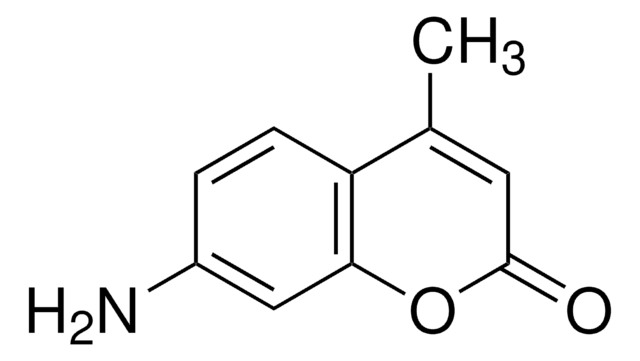
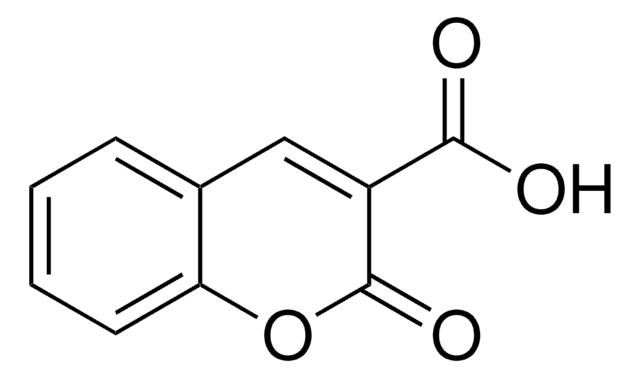
3-Cyanoumbelliferone
BioReagent, suitable for fluorescence, ≥98.0% (TLC)
Synonim(y):
3-Cyano-7-hydroxycoumarin
About This Item
Polecane produkty
linia produktu
BioReagent
Poziom jakości
Próba
≥98.0% (TLC)
Postać
powder
mp
≥250 °C (lit.)
rozpuszczalność
DMF: soluble
alcohols: soluble
fluorescencja
λex 408 nm; λem 450 nm in methanol
przydatność
suitable for fluorescence
ciąg SMILES
Oc1ccc2C=C(C#N)C(=O)Oc2c1
InChI
1S/C10H5NO3/c11-5-7-3-6-1-2-8(12)4-9(6)14-10(7)13/h1-4,12H
Klucz InChI
IJQYTHQDUDCJEQ-UHFFFAOYSA-N
informacje o genach
human ... MIF(4282)
Zastosowanie
Opakowanie
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organy docelowe
Respiratory system
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
dust mask type N95 (US), Eyeshields, Gloves
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Nitric oxide (NO) as a signal transporter in neurons, endothelial cells and in the immune system.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej