Key Documents
T1500000
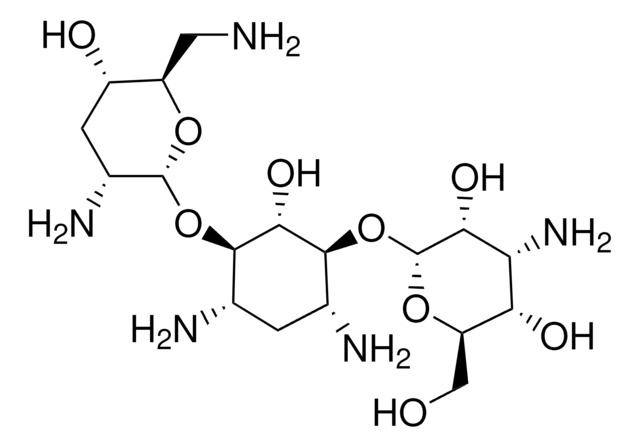
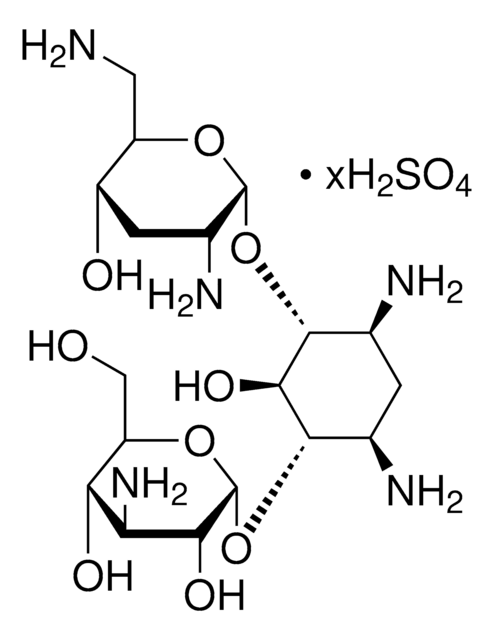
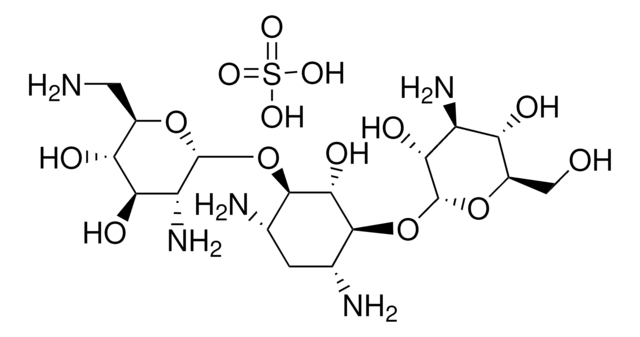
Tobramycin
European Pharmacopoeia (EP) Reference Standard
Synonim(y):
Nebramycin Factor 6, O-[3-Amino-3-deoxy-α-D-glucopyranosyl-(1→6)]-O-[2,6-diamino-2,3,6-trideoxy-α-D-ribohexopyranosyl-(1→4)]-2-deoxy-D-streptamine
About This Item
Polecane produkty
rodzina API
tobramycin
producent / nazwa handlowa
EDQM
Zastosowanie
pharmaceutical (small molecule)
format
neat
temp. przechowywania
2-8°C
ciąg SMILES
NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@@H](N)[C@H]3O)[C@H]2O)[C@H](N)C[C@@H]1O
InChI
1S/C18H37N5O9/c19-3-9-8(25)2-7(22)17(29-9)31-15-5(20)1-6(21)16(14(15)28)32-18-13(27)11(23)12(26)10(4-24)30-18/h5-18,24-28H,1-4,19-23H2/t5-,6+,7+,8-,9+,10+,11+,12+,13+,14-,15+,16-,17+,18+/m0/s1
Klucz InChI
NLVFBUXFDBBNBW-SNGYORCQSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Opis ogólny
Zastosowanie
Działania biochem./fizjol.
Mode of Action: Binds to 70S ribosomal subunit; inhibits translocation; elicits miscoding.
Spectrum of Activity: Gram negative bacteria. Not effective against Enterococci.
Opakowanie
Inne uwagi
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Repr. 2
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Przepraszamy, ale COA dla tego produktu nie jest aktualnie dostępny online.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej