SMB01361
Phosphoramide mustard cyclohexylamine
≥95% (HPLC)
Synonim(y):
N,N-Bis(2-Chloroethyl)phosphorodiamidic acid, cyclohexylamine, Friedman acid, cyclohexylamine, PAM cyclohexylamine, PM cyclohexylamine, PMC, Phosphamide mustard, Cyclohexylamine, Phosphorodiamidic mustard, Cyclohexylamine
About This Item
Polecane produkty
Poziom jakości
Próba
≥95% (HPLC)
Postać
solid
temp. przechowywania
-10 to -25°C
ciąg SMILES
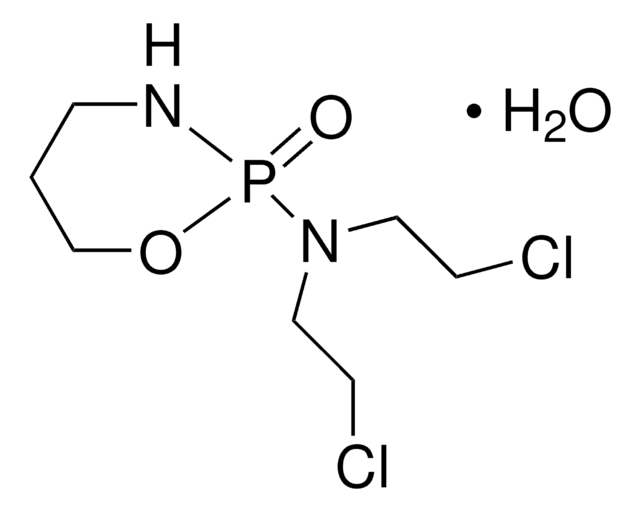
ClCCN(P(N)(O)=O)CCCl.NC1CCCCC1
InChI
1S/C6H13N.C4H11Cl2N2O2P/c7-6-4-2-1-3-5-6;5-1-3-8(4-2-6)11(7,9)10/h6H,1-5,7H2;1-4H2,(H3,7,9,10)
Klucz InChI
BGTIPRUDEMNRIP-UHFFFAOYSA-N
Opis ogólny
Zastosowanie
Działania biochem./fizjol.
Cechy i korzyści
- High quality compound suitable for multiple research applications
- Compatible with a wide variety of chromatographic and spectrometry techniques
Inne uwagi
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Przepraszamy, ale COA dla tego produktu nie jest aktualnie dostępny online.
Proszę o kontakt, jeśli potrzebna jest pomoc Obsługa Klienta
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej