Kluczowe dokumenty
31102
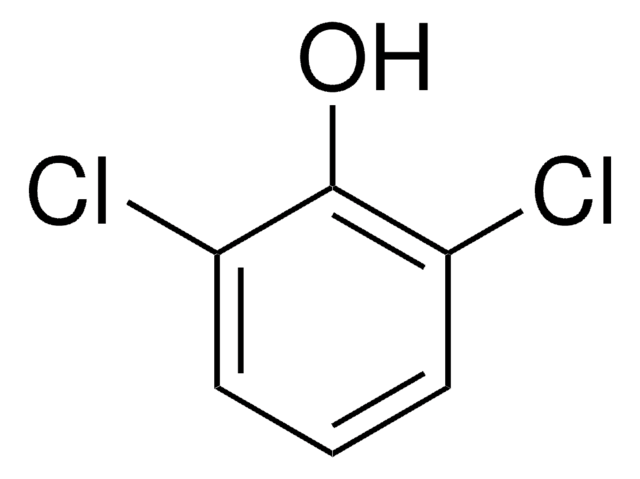
2,6-Dichlorophenol
PESTANAL®, analytical standard
About This Item
Polecane produkty
klasa czystości
analytical standard
linia produktu
PESTANAL®
okres trwałości
limited shelf life, expiry date on the label
metody
HPLC: suitable
gas chromatography (GC): suitable
bp
218-220 °C (lit.)
mp
64-66 °C (lit.)
Zastosowanie
agriculture
environmental
Format
neat
ciąg SMILES
Oc1c(Cl)cccc1Cl
InChI
1S/C6H4Cl2O/c7-4-2-1-3-5(8)6(4)9/h1-3,9H
Klucz InChI
HOLHYSJJBXSLMV-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Opis ogólny
Zastosowanie
- To study the kinetics, performance, and mechanism of the oxidative degradation of 2,6-dichlorophenol (2,6-DCP) by ferrate (VI) (Fe(VI))
- Removal of 2,6-dichlorophenol in water by copper oxide (CuO) activated peroxymonosulfate as catalyst
- Removal of 2,6-dichlorophenol by adsorption with activated polypropylene nanofiber
- Degradation of 2,6-dichlorophenol by Fe-doped titanium oxide(TiO2) sonophotocatalytic process
- Determination of 2,6-dichlorophenol by surface-enhanced Raman scattering (SERS) with molecular imprinting
Informacje prawne
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Skin Corr. 1B
Kod klasy składowania
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Klasa zagrożenia wodnego (WGK)
WGK 2
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Nie widzisz odpowiedniej wersji?
Jeśli potrzebujesz konkretnej wersji, możesz wyszukać konkretny certyfikat według numeru partii lub serii.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej