CC050
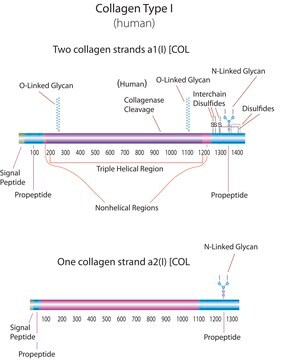
Human Collagen Type I
from human placenta, liquid, 1 mg/mL, suitable for cell culture, used for gel formation
Synonim(y):
Human Collagen
About This Item
Polecane produkty
Nazwa produktu
Human Collagen Type I,
pochodzenie biologiczne
human
Poziom jakości
Próba
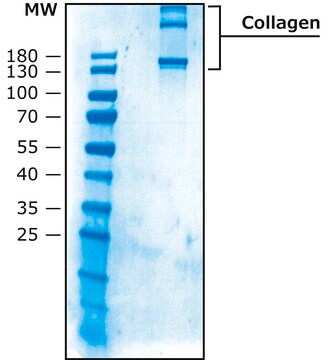
>90% (collagen type I, SDS-PAGE)
Formularz
liquid
producent / nazwa handlowa
Chemicon®
stężenie
1 mg/mL
metody
cell culture | mammalian: suitable
zanieczyszczenia
<1% collagen type II,IV-VI & non-collagen proteins.
<10% collagen type III
moc wejściowa
sample type pancreatic stem cell(s)
sample type mesenchymal stem cell(s)
sample type epithelial cells
sample type induced pluripotent stem cell(s)
sample type neural stem cell(s)
sample type: human embryonic stem cell(s)
sample type hematopoietic stem cell(s)
rozpuszczalność
water: soluble at 20 °C
numer dostępu NCBI
numer dostępu UniProt
Specyficzność wiązania
Peptide Source: Elastin
Peptide Source: Fibronectin
Warunki transportu
dry ice
temp. przechowywania
−20°C
informacje o genach
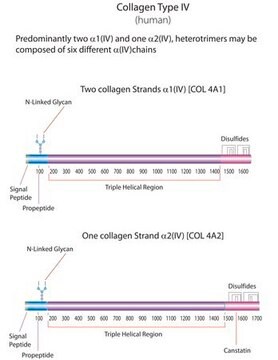
human ... COL1A1(1277)
Opis ogólny
Zastosowanie
- as a control in the 2B4 nuclear factor of activated T-cells (NFAT)–GFP reporter cell assay, where its interaction with reporter cells can be evaluated for nuclear factor of activated T-cells (NFAT) promoter-driven GFP expression
- for coating glass slides in dynamic binding assays to create a substrate for the specific binding and study of platelets and conjugates in flow channels
- as a protein standard in histological analysis of lung tissue samples, providing a reference for the composition and characterization of extracellular matrix (ECM) components
Działania biochem./fizjol.
Postać fizyczna
Komentarz do analizy
Informacje prawne
Oświadczenie o zrzeczeniu się odpowiedzialności
Kod klasy składowania
12 - Non Combustible Liquids
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
Białka macierzy zewnątrzkomórkowej, takie jak laminina, kolagen i fibronektyna, mogą być stosowane jako podłoża do mocowania komórek w hodowli komórkowej.
Extracellular matrix proteins such as laminin, collagen, and fibronectin can be used as cell attachment substrates in cell culture.
Protokoły
Ta strona obejmuje protokoły powlekania ECM opracowane dla czterech rodzajów ECM na wkładkach Millicell®-CM, kolagenu typu 1, fibronektyny, lamininy i matrigelu.
Powiązane treści
This page covers the ECM coating protocols developed for four types of ECMs on Millicell®-CM inserts, Collagen Type 1, Fibronectin, Laminin, and Matrigel.
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej