324725
Endoglycosidase F1, Elizabethkingia meningosepticum, Recombinant, E. coli
Endoglycosidase F1, Elizabethkingia meningosepticum, Recombinant, E. coli cleaves asparagine-linked or free oligomannose and hybrid. Suitable for deglycosylation of native proteins.
Synonim(y):
Endo-β-N-acetylglucosaminidase F1, Endo F1
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Polecane produkty
rekombinowane
expressed in E. coli
Poziom jakości
białko sprzężone
(N-linked)
Postać
liquid
aktywność właściwa
≥16 units/mg protein
≥17 units/mL
producent / nazwa handlowa
Calbiochem®
warunki przechowywania
do not freeze
obecność zanieczyszczeń
Proteases, none detected
Warunki transportu
wet ice
temp. przechowywania
2-8°C
Powiązane kategorie
Opis ogólny
Note: 1 mU = 1 milliunit.
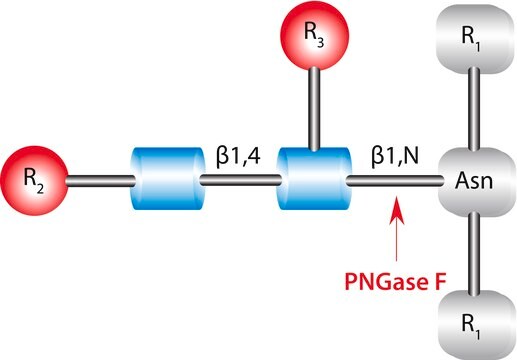
Recombinant, Elizabethkingia meningosepticum endoglycosidase F1 expressed in E. coli. Cleaves asparagine-linked or free oligomannose and hybrid, but not complex oligosaccharides. Core fucosylation reduces activity by 50 fold. Endo F1 will hydrolyze sulfate containing high mannose chains. It cleaves between the two N-acetylglucosamine residues in the diacetylchitobiose core of the oligosaccharide generating a truncated sugar molecule with one N-acetylglucosamine residue remaining on the asparagine. Less sensitive to protein conformation than N-Glycosidase F (Cat. No. 362185) and therefore is more suitable for deglycosylation of native proteins.
Recombinant, Elizabethkingia meningosepticum endoglycosidase F1 expressed in E. coli. Cleaves asparagine-linked or free oligomannose and hybrid, but not complex oligosaccharides. Core fucosylation reduces activity by 50 fold. Endo F1 will hydrolyze sulfate containing high mannose chains. It cleaves between the two N-acetylglucosamine residues in the diacetylchitobiose core of the oligosaccharide generating a truncated sugar molecule with one N-acetylglucosamine residue remaining on the asparagine. Less sensitive to protein conformation than N-Glycosidase F (Cat. No. 362185), and therefore is more suitable for deglycosylation of native proteins.
Ostrzeżenie
Toxicity: Standard Handling (A)
Definicja jednostki
One unit is defined as the amount of enzyme that will release N-linked oligosaccharides from 1.0 µmol denatured ribonuclease B per min at 37°C, pH 5.5.
Postać fizyczna
In 20 mM Tris-HCl, pH 7.5.
Inne uwagi
Tarentino, A.L., and Plummer, T.H. 1994. Methods Enzymol. 230, 44.
Tarentino, A.L., et al. 1992. J. Biol. Chem. 267, 3868.
Trimble, R.B., and Tarentino, A.L. 1991. J. Biol. Chem. 266, 1646.
Tarentino, A.L., et al. 1992. J. Biol. Chem. 267, 3868.
Trimble, R.B., and Tarentino, A.L. 1991. J. Biol. Chem. 266, 1646.
Informacje prawne
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Ta strona może zawierać tekst przetłumaczony maszynowo.
Kod klasy składowania
10 - Combustible liquids
Klasa zagrożenia wodnego (WGK)
WGK 1
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Certyfikaty analizy (CoA)
Poszukaj Certyfikaty analizy (CoA), wpisując numer partii/serii produktów. Numery serii i partii można znaleźć na etykiecie produktu po słowach „seria” lub „partia”.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
A L Tarentino et al.
The Journal of biological chemistry, 267(6), 3868-3872 (1992-03-06)
A full-length insert for the endo-beta-N-acetylglucosaminidase (Endo) F1 gene was located on a 2,200-base pair EcoRI fragment of genomic DNA and cloned into the plasmid vector Bluescript. Transformed Escherichia coli cells expressed Endo F1 activity very well, but the enzyme
R B Trimble et al.
The Journal of biological chemistry, 266(3), 1646-1651 (1991-01-25)
Flavobacterium meningosepticum endo-beta-acetyl-glucosaminidase F preparations have been resolved by hydrophobic interaction chromatography on TSK-butyl resin into at least three activities designated endo F1, endo F2 and endo F3 each with a unique substrate specificity. The 32-kDa endo F1 protein is
Enzymatic deglycosylation of asparagine-linked glycans: purification, properties, and specificity of oligosaccharide-cleaving enzymes from Flavobacterium meningosepticum.
A L Tarentino et al.
Methods in enzymology, 230, 44-57 (1994-01-01)
Thapakorn Jaroentomeechai et al.
Nature communications, 13(1), 6325-6325 (2022-10-25)
The ability to reconstitute natural glycosylation pathways or prototype entirely new ones from scratch is hampered by the limited availability of functional glycoenzymes, many of which are membrane proteins that fail to express in heterologous hosts. Here, we describe a
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej