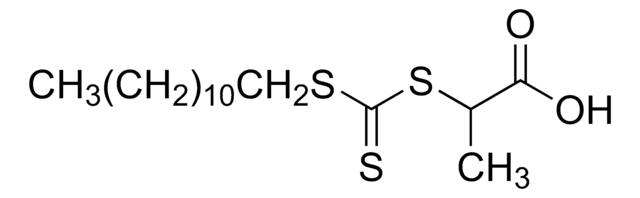
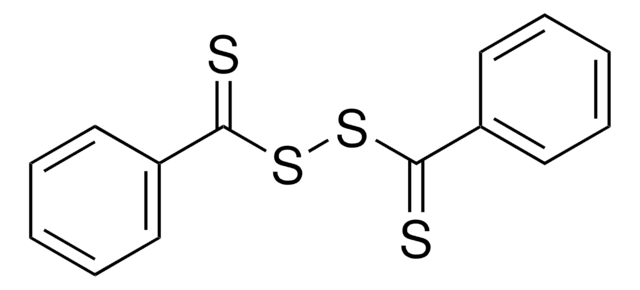
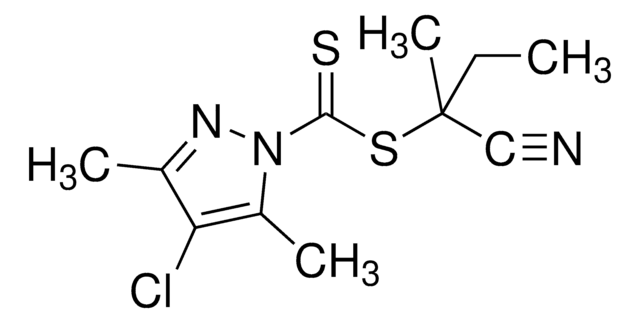
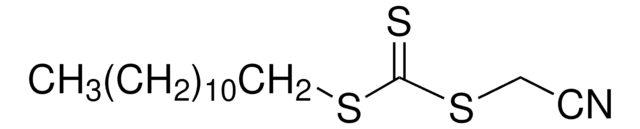Kluczowe dokumenty
722987
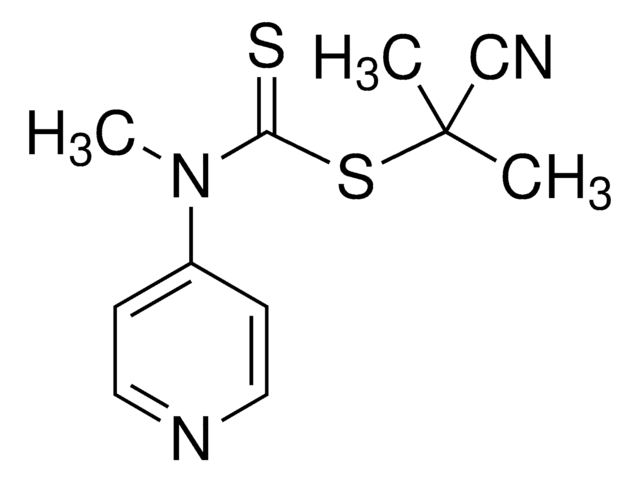
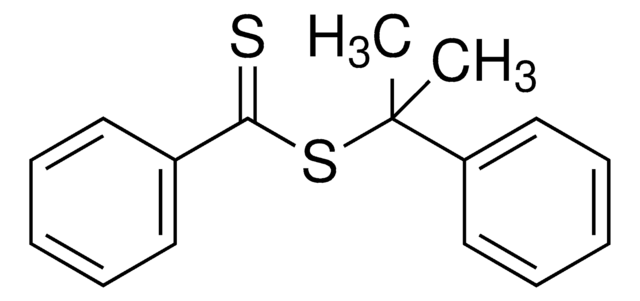
2-Cyano-2-propyl benzodithioate
>97% (HPLC)
Synonim(y):
2-Cyanopropan-2-yl benzodithioate
About This Item
Polecane produkty
Poziom jakości
Próba
>97% (HPLC)
Formularz
solid or liquid
współczynnik refrakcji
n20/D 1.621
mp
28-31 °C
gęstość
1.146 g/mL at 25 °C
temp. przechowywania
2-8°C
ciąg SMILES
CC(C)(SC(=S)c1ccccc1)C#N
InChI
1S/C11H11NS2/c1-11(2,8-12)14-10(13)9-6-4-3-5-7-9/h3-7H,1-2H3
Klucz InChI
IDSLBLWCPSAZBL-UHFFFAOYSA-N
Powiązane kategorie
Opis ogólny
Zastosowanie
Hasło ostrzegawcze
Warning
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Skin Sens. 1
Kod klasy składowania
10 - Combustible liquids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
>230.0 °F - closed cup
Temperatura zapłonu (°C)
> 110 °C - closed cup
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
Produkty
A series of polymerization were carried out using RAFT agents and monomers yielding well-defined polymers with narrow molecular weight distributions. The process allows radical-initiated growing polymer chains to degeneratively transfer reactivity from one to another through the use of key functional groups (dithioesters, trithiocarbonates, xanthates and dithiocarbamates). RAFT agents help to minimize out-of-control growth and prevent unwanted termination events from occurring, effectively controlling polymer properties like molecular weight and polydispersity. RAFT agents are commercially available. RAFT does not use any cytotoxic heavy metal components (unlike ATRP).
RAFT (Reversible Addition Fragmentation chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
Over the past two decades, the rapid advance of controlled living polymerization (CLP) techniques.
The modification of biomacromolecules, such as peptides and proteins, through the attachment of synthetic polymers has led to a new family of highly advanced biomaterials with enhanced properties.
Protokoły
RAFT (Reversible Addition-Fragmentation chain Transfer) is a form of living radical polymerization involving conventional free radical polymerization of a substituted monomer in the presence of a suitable chain transfer (RAFT) reagent.
Polimeryzacja RAFT oferuje precyzyjną kontrolę, umożliwiając dostosowaną syntezę złożonych struktur polimerowych.
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
Sigma-Aldrich presents an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 722987-1G | 4061837817229 |
| 722987-5G | 4061832860961 |
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej
![4-Cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanoic acid 97% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/204/925/30ae6ca0-5b0b-4963-a061-7e5e3d1a85af/640/30ae6ca0-5b0b-4963-a061-7e5e3d1a85af.png)
![4-Cyano-4-[(dodecylsulfanylthiocarbonyl)sulfanyl]pentanol](/deepweb/assets/sigmaaldrich/product/structures/839/520/64c23004-f340-460f-a379-8670a35d0433/640/64c23004-f340-460f-a379-8670a35d0433.png)