Wszystkie zdjęcia(2)
Key Documents
670774
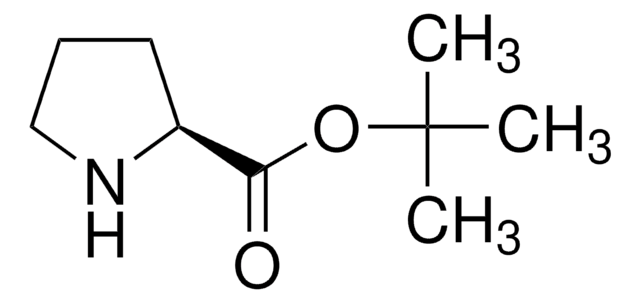
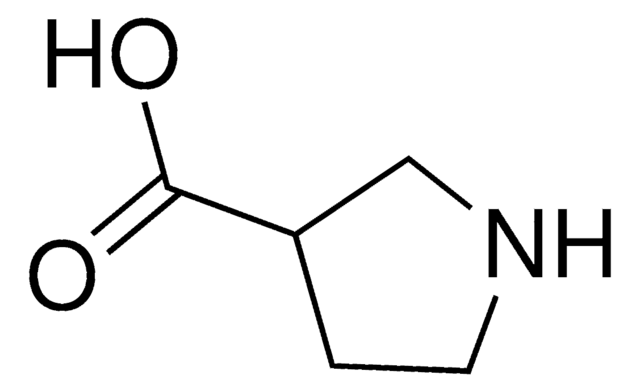
(R)-(−)-Pyrrolidine-3-carboxylic acid
≥99.0% (NT)
Synonim(y):
(R)-β-Proline
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
C5H9NO2
Numer CAS:
Masa cząsteczkowa:
115.13
Beilstein:
5496144
Numer MDL:
Kod UNSPSC:
12352200
Identyfikator substancji w PubChem:
Polecane produkty
Próba
≥99.0% (NT)
Postać
solid
aktywność optyczna
[α]/D -20.5±1.5°, c = 2 in H2O
przydatność reakcji
reaction type: solution phase peptide synthesis
Zastosowanie
peptide synthesis
temp. przechowywania
2-8°C
ciąg SMILES
OC(=O)[C@@H]1CCNC1
InChI
1S/C5H9NO2/c7-5(8)4-1-2-6-3-4/h4,6H,1-3H2,(H,7,8)/t4-/m1/s1
Klucz InChI
JAEIBKXSIXOLOL-SCSAIBSYSA-N
Opakowanie
Bottomless glass bottle. Contents are inside inserted fused cone.
This page may contain text that has been machine translated.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
David Steer et al.
Biochemistry, 41(35), 10819-10826 (2002-08-28)
The enzyme EC 3.4.24.15 (EP 24.15) is a zinc metalloendopeptidase whose precise function in vivo remains unknown but is thought to participate in the regulated metabolism of a number of specific neuropeptides. The lack of stable and selective inhibitors has
Wesley R R Harker et al.
Organic & biomolecular chemistry, 10(7), 1406-1410 (2012-01-05)
α-Alkyl β-amino esters are available in high diastereoselectivity through a silicon-free Claisen enolate [3,3]-sigmatropic rearrangement of enamide esters. Optimisation studies have probed the crucial role of the initial enolisation and the nature of the enamide N-centre. The demonstration of chirality
Haile Zhang et al.
Journal of the American Chemical Society, 130(3), 875-886 (2008-01-01)
The development of enantioselective anti-selective Mannich-type reactions of aldehydes and ketones with imines catalyzed by 3-pyrrolidinecarboxylic acid and related pyrrolidine derivatives is reported in detail. Both (3R,5R)-5-methyl-3-pyrrolidinecarboxylic acid and (R)-3-pyrrolidinecarboxylic acid efficiently catalyzed the reactions of aldehydes with alpha-imino esters
Cody Timmons et al.
The Journal of organic chemistry, 70(19), 7634-7639 (2005-09-10)
[reaction: see text] A new halo-Mannich-type reaction is reported using cyclopropyl carbonyl-derived enolates and sulfonyl-protected imines. Chiral oxazolidinones auxiliaries were found to be effective for completely controlling the stereochemistry of the products. Variations in the oxazolidinone, protecting group, and imine
Souvik Banerjee et al.
The Journal of organic chemistry, 77(23), 10925-10930 (2012-11-07)
A straightforward stereoselective and enantiodivergent cyclization strategy for the construction of γ-lactams is described. The cyclization strategy makes use of chiral malonic esters prepared from enantiomerically enriched monoesters of disubstituted malonic acid. The cyclization occurs with the selective displacement of
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej

![(S)-α,α-Bis[3,5-bis(trifluoromethyl)phenyl]-2-pyrrolidinemethanol ≥99.0%](/deepweb/assets/sigmaaldrich/product/structures/201/440/11d18670-8609-4657-bb4b-af6c424f8791/640/11d18670-8609-4657-bb4b-af6c424f8791.png)