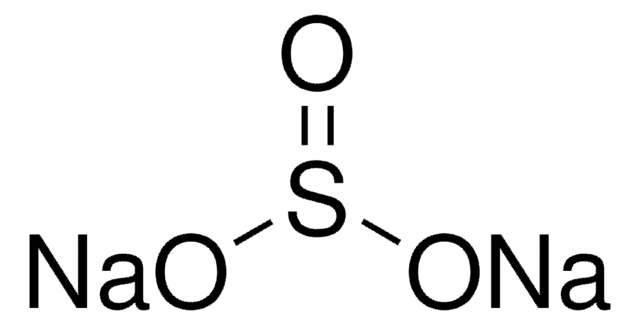
Kluczowe dokumenty
407410
Sodium sulfide
Synonim(y):
Disodium sulfide
About This Item
Polecane produkty
Postać
solid
Poziom jakości
przydatność reakcji
reagent type: catalyst
core: sodium
mp
950 °C (lit.)
gęstość
1.86 g/mL at 25 °C (lit.)
temp. przechowywania
2-8°C
ciąg SMILES
[Na]S[Na]
InChI
1S/2Na.S
Klucz InChI
CXPWOVUZRAFMDA-UHFFFAOYSA-N
Opis ogólny
Zastosowanie
Cechy i korzyści
- Quality Guaranteed - Quality-tested to be 98% pure for consistent results.
- Available to scale up: bulk and pilot scale
Hasło ostrzegawcze
Danger
Zwroty wskazujące rodzaj zagrożenia
Zwroty wskazujące środki ostrożności
Klasyfikacja zagrożeń
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Acute 1 - Eye Dam. 1 - Met. Corr. 1 - Self-heat. 1 - Skin Corr. 1B
Zagrożenia dodatkowe
Kod klasy składowania
4.2 - Pyrophoric and self-heating hazardous materials
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Wybierz jedną z najnowszych wersji:
Certyfikaty analizy (CoA)
Nie widzisz odpowiedniej wersji?
Jeśli potrzebujesz konkretnej wersji, możesz wyszukać konkretny certyfikat według numeru partii lub serii.
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Klienci oglądali również te produkty
endothelial function in a canine model of cardiopulmonary
bypass
Produkty
Noble-Metal Nanostructures with Controlled Morphologies
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej



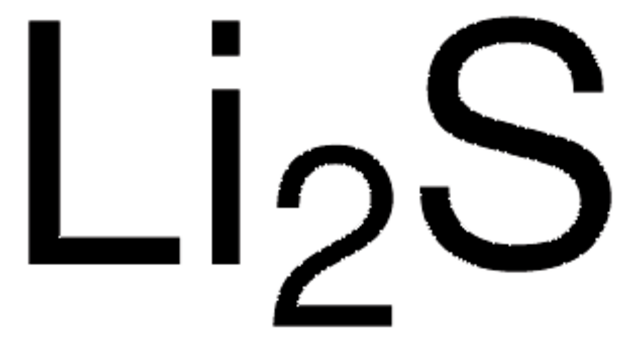

![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)