Wszystkie zdjęcia(2)
Kluczowe dokumenty
311073
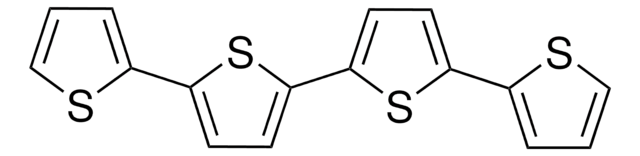
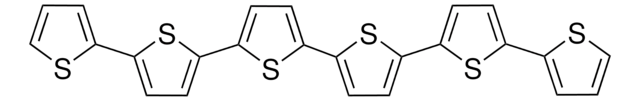
2,2′:5′,2′′-Terthiophene
99%
Synonim(y):
α-Terthienyl, 2,5-Di(2-thienyl)thiophene
Zaloguj sięWyświetlanie cen organizacyjnych i kontraktowych
About This Item
Wzór empiryczny (zapis Hilla):
C12H8S3
Numer CAS:
Masa cząsteczkowa:
248.39
Beilstein:
178604
Numer MDL:
Kod UNSPSC:
12352103
Identyfikator substancji w PubChem:
NACRES:
NA.23
Polecane produkty
Próba
99%
mp
93-95 °C (lit.)
ciąg SMILES
c1csc(c1)-c2ccc(s2)-c3cccs3
InChI
1S/C12H8S3/c1-3-9(13-7-1)11-5-6-12(15-11)10-4-2-8-14-10/h1-8H
Klucz InChI
KXSFECAJUBPPFE-UHFFFAOYSA-N
Szukasz podobnych produktów? Odwiedź Przewodnik dotyczący porównywania produktów
Powiązane kategorie
Opis ogólny
2,2′:5′,2′′-Terthiophene (TTh) may be prepared by nickel catalysed coupling reaction of grignard′s reagent derived from 2-bromothiophene and magnesium. It generates singlet oxygen. In nature, it is found in the floral extract of Tagetes minuta and Echinops grijisii. It is known to be toxic to mosquitoes. It also exihibits antifungal activity.
Zastosowanie
3T can be combined with 3,4-ethylenedioxythiophene (EDOT) in a tetrabutylammonium perchlorate solution for use as an electrochromic copolymer for a wide range of applications like photovoltaics and polymer light emitting diodes (LEDs). It can also be used to form metal-organic based thin films with metals like aluminum, silver, and calcium which can potentially be used for optoelectronics based applications.
Electrochemical copolymerization of carbazole and TTh in sodium perchlorate/acetonitrile was reported. Electrochromic copolymer based on TTh and 3, 4-ethylenedioxythiophene has been reported. TTh acts as a monomer precursor for polythiophene and as a dopant for polycarbonate. It may function as a photosensitizer.
Ta strona może zawierać tekst przetłumaczony maszynowo.
Kod klasy składowania
11 - Combustible Solids
Klasa zagrożenia wodnego (WGK)
WGK 3
Temperatura zapłonu (°F)
Not applicable
Temperatura zapłonu (°C)
Not applicable
Środki ochrony indywidualnej
Eyeshields, Gloves, type N95 (US)
Wybierz jedną z najnowszych wersji:
Masz już ten produkt?
Dokumenty związane z niedawno zakupionymi produktami zostały zamieszczone w Bibliotece dokumentów.
Synthesis and characterization of an electrochromic copolymer based on 2, 2′ : 5′ , 2″ -terthiophene and 3, 4-ethylenedioxythiophene
Ahmed MS, et al.
Applied Nanoscience, 2(2), 133-141 (2012)
Man Jae Park et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(7), 2056-2062 (2012-01-18)
Excess-electron transfer (EET) in DNA has attracted wide attention owing to its close relation to DNA repair and nanowires. To clarify the dynamics of EET in DNA, a photosensitizing electron donor that can donate an excess electron to a variety
Journal of Non-Crystalline Solids, 164, 1263-1263 (1993)
Hui-Bog Noh et al.
Biomaterials, 33(9), 2600-2607 (2012-01-03)
A highly sensitive in vivo biosensor for glutathione disulfide (GSSG) is developed using covalently immobilized-glutathione reductase (GR) and -β-nicotinamide adenine dinucleotide phosphate (NADPH) on gold nanoparticles deposited on poly[2,2':5',2″-terthiophene-3'-(p-benzoic acid)] (polyTTBA). The fabricated biosensor was characterized with SEM, TEM, XPS
Synthesis and characterization of an electrochromic copolymer based on 2,2':5',"-terthiophene and 3,4-ethylenedioxythiophene
Ahmed MS, et al.
Applied Nanoscience, 2, 133-141 (2012)
Nasz zespół naukowców ma doświadczenie we wszystkich obszarach badań, w tym w naukach przyrodniczych, materiałoznawstwie, syntezie chemicznej, chromatografii, analityce i wielu innych dziedzinach.
Skontaktuj się z zespołem ds. pomocy technicznej


![Benzo[1,2-b:4,5-b′]dithiophene-4,8-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/418/544/b7faac0b-ad09-4b42-a9fa-aeb38017a39e/640/b7faac0b-ad09-4b42-a9fa-aeb38017a39e.png)