About This Item
추천 제품
Quality Level
분석
≥97% (HPLC)
양식
solid
색상
white
mp
127-132 °C (lit.)
solubility
DMSO: ≥20 mg/mL
주관자
Merck & Co., Inc., Kenilworth, NJ, U.S.
저장 온도
2-8°C
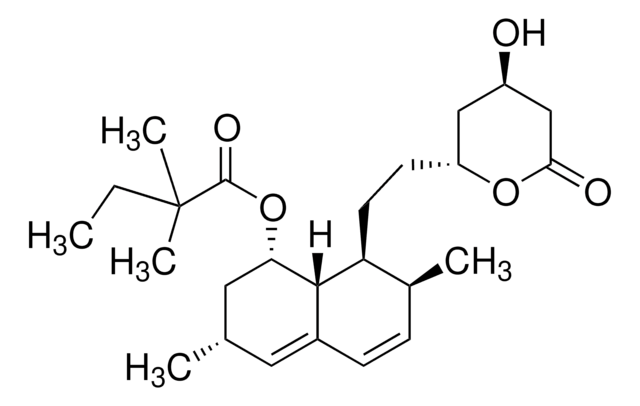
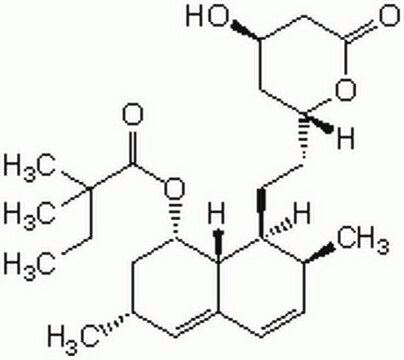
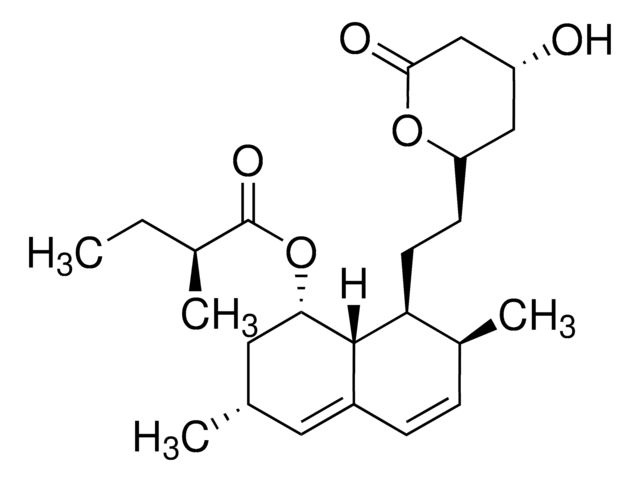
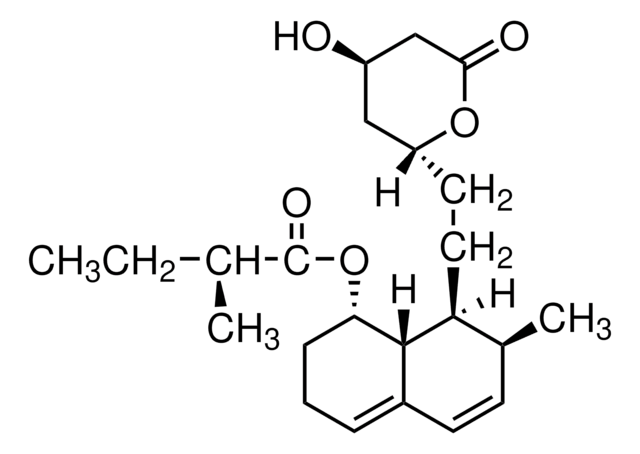
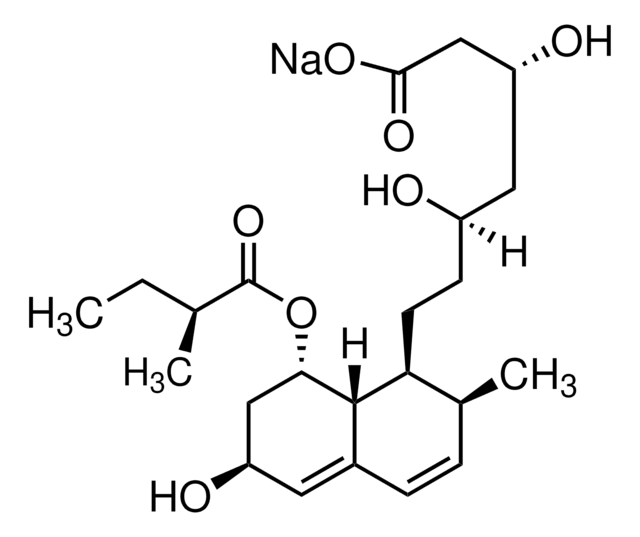
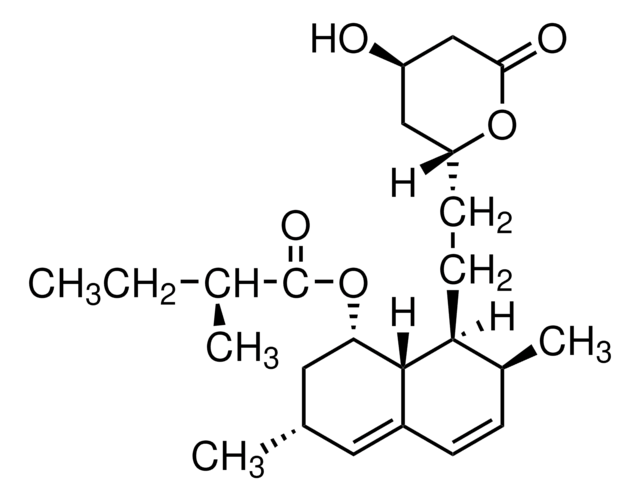
SMILES string
[H][C@]12[C@H](C[C@@H](C)C=C1C=C[C@H](C)[C@@H]2CC[C@@H]3C[C@@H](O)CC(=O)O3)OC(=O)C(C)(C)CC
InChI
1S/C25H38O5/c1-6-25(4,5)24(28)30-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-19-13-18(26)14-22(27)29-19/h7-8,11,15-16,18-21,23,26H,6,9-10,12-14H2,1-5H3/t15-,16-,18+,19+,20-,21-,23-/m0/s1
InChI key
RYMZZMVNJRMUDD-HGQWONQESA-N
유전자 정보
human ... HMGCR(3156)
rat ... Hmgcr(25675)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
- as an inhibitor of HMG CoA reductase (HMGCR)
- to study its effects on epithelial to mesenchymal transition (EMT) and the prognosis of patients with lung adenocarcinoma
- in in vivo studies to test its effect on brain tumor−initiating cells (BTIC) viability and cell proliferation
- to study the role of adenosine triphosphate (ATP)-binding cassette transporter A7 in phagocytosis of Jurkat cells
- to study the effect on endothelial dysfunction and inflammation in mice
생화학적/생리학적 작용
특징 및 장점
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
문서
The amount of cholesterol that is synthesized in the liver is tightly regulated by dietary cholesterol levels. LDL receptors regulate the cellular transport of lipid rich low density lipoprotein (LDL) particles.
Terpenes comprise the largest and most diverse class of secondary metabolites; approximately 55,000 compounds have been identified to date.
Randomized controlled clinical studies have suggested 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are effective in both primary and secondary prevention of cardiovascular disease (CVD) events.
Antilipemic Agents
관련 콘텐츠
Discover Bioactive Small Molecules for Lipid Signaling Research
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.