추천 제품
Grade
certified reference material
pharmaceutical secondary standard
Quality Level
Agency
traceable to Ph. Eur. L0790000
traceable to USP 1370600
API family
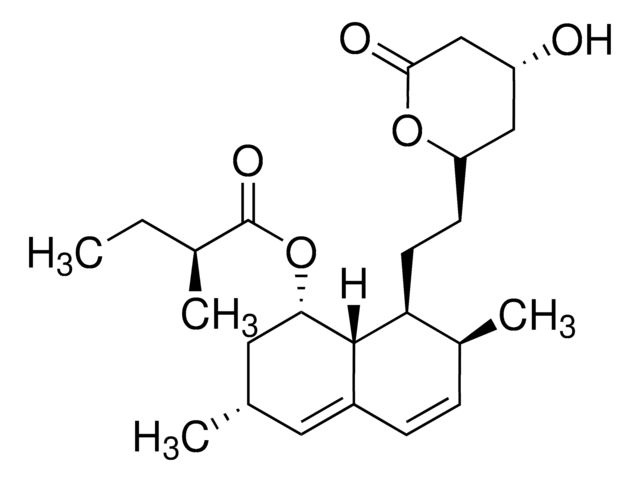
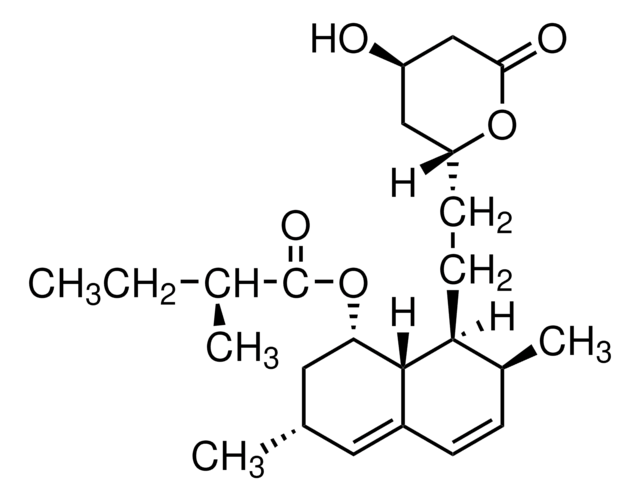
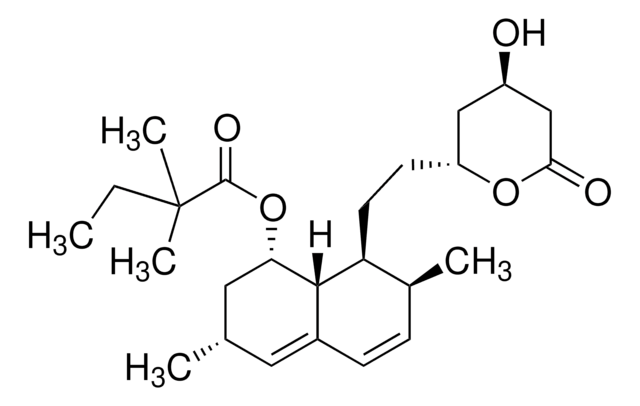
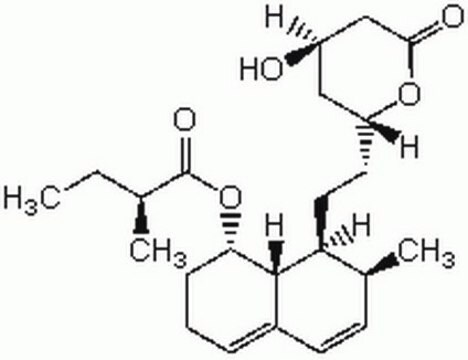
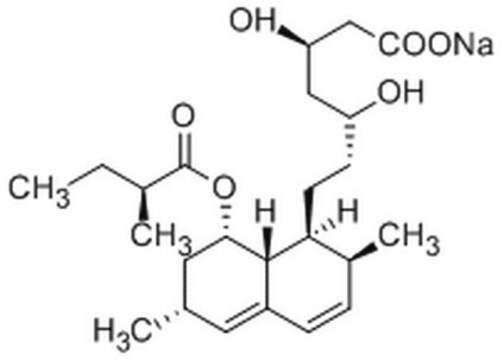
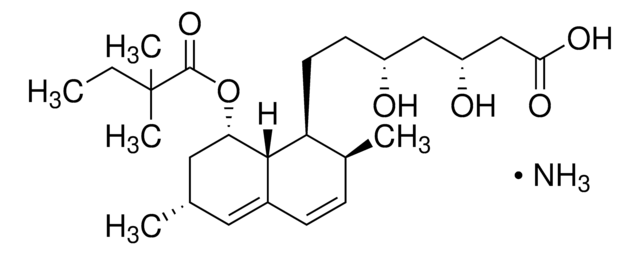
lovastatin
CofA
current certificate can be downloaded
기술
HPLC: suitable
gas chromatography (GC): suitable
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
-10 to -25°C
SMILES string
O1[C@@H](C[C@H](CC1=O)O)CC[C@@H]2[C@H]3[C@H](C[C@H](C=C3C=C[C@@H]2C)C)OC(=O)[C@H](CC)C
InChI
1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19+,20-,21-,23-/m0/s1
InChI key
PCZOHLXUXFIOCF-BXMDZJJMSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Pharmaceutical secondary standard for application in quality control. Provides pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
애플리케이션
분석 메모
기타 정보
각주
관련 제품
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Carc. 2 - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.