추천 제품
분석
≥80%
Quality Level
양식
powder
작용기
epoxy
배송 상태
ambient
저장 온도
room temp
SMILES string
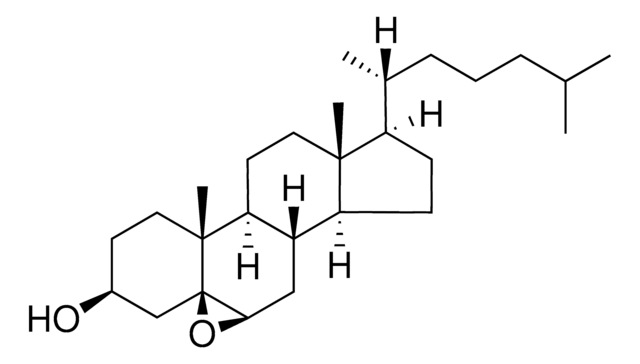
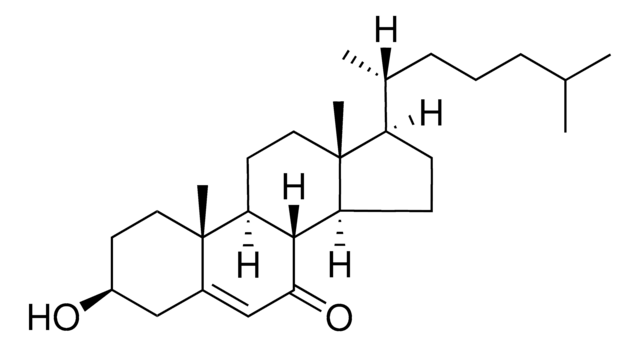
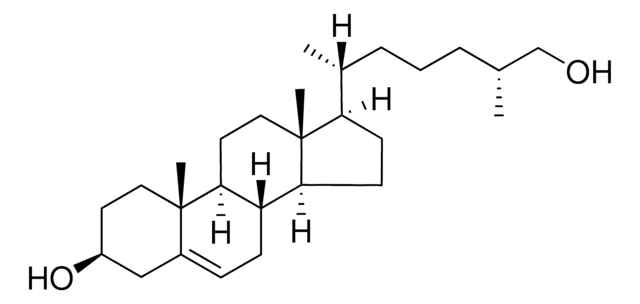
[H][C@@]12[C@]([C@](CC[C@H](O)C3)(C)[C@@]3(O4)[C@@H]4C2)([H])CC[C@@]5(C)[C@@]1([H])CC[C@]5([H])[C@]([H])(C)CCCC(C)C
InChI
1S/C27H46O2/c1-17(2)7-6-8-18(3)21-9-10-22-20-15-24-27(29-24)16-19(28)11-14-26(27,5)23(20)12-13-25(21,22)4/h17-24,28H,6-16H2,1-5H3/t18-,19+,20+,21-,22+,23+,24+,25-,26-,27+/m1/s1
InChI key
PRYIJAGAEJZDBO-ZEQHCUNVSA-N
애플리케이션
Cholesterol 5α, 6α-epoxide was incorporated in culture medium of human arterial endothelial cells to study oxysterol-induced toxicity.
생화학적/생리학적 작용
Cholesterol 5α, 6α-epoxide is an oxysterol, a cholesterol derivative by auto-oxidation. Oxysterols are non-genomic regulators of cholesterol homeostasis. The biological effects include protein prenylation, apoptosis, modulation of sphingolipid metabolism and platelet aggregation. Oxysterols bind to liver X receptors, modulate cholesterol efflux and decrease the uptake of cholesterol by the cells.
제조 메모
Cholesterol 5α, 6α-epoxide yields clear, colorless solution in chloroform at 50 mg/ml.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Anne Vejux et al.
Histochemistry and cell biology, 127(6), 609-624 (2007-01-18)
Oxysterols, mainly those oxidized at the C7 position, induce a complex mode of cell death exhibiting some characteristics of apoptosis associated with a rapid induction of lipid rich multilamellar cytoplasmic structures (myelin figures) observed in various pathologies including atherosclerosis. The
Chisato Ishimaru et al.
Lipids, 43(4), 373-382 (2008-01-25)
This paper describes the inhibitory activities of cholesterol derivatives such as cholesterol, sodium cholesteryl sulfate, cholesteryl-5alpha, 6alpha-epoxide, cholesteryl chloride, cholesteryl bromide, and cholesteryl hemisuccinate (compounds 1-6, respectively) against DNA polymerase (pol), DNA topoisomerase (topo), and human cancer cell growth. Among
Y W Cheng et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 43(4), 617-622 (2005-02-22)
The mutagenicity of oxysterols, cholesterol-3beta,5alpha,6beta-triol (alpha-Triol), 7-keto-cholesterol (7-Keto) and cholesterol-5alpha,6alpha-epoxide (alpha-Epox) were examined by the Ames method and chromosome aberration test in this study. Only alpha-Triol concentration-dependently caused an increase of bacterial revertants in the absence of metabolic activating enzymes
I Spyridopoulos et al.
Arteriosclerosis, thrombosis, and vascular biology, 21(3), 439-444 (2001-03-07)
Controversy exists about the net effect of alcohol on atherogenesis. A protective effect is assumed, especially from the tannins and phenolic compounds in red wine, owing to their inhibition of low density lipoprotein (LDL) oxidation. However, increased atherogenesis occurs in
Lisa Ryan et al.
The British journal of nutrition, 94(4), 519-525 (2005-10-04)
Oxysterols are oxygenated derivatives of cholesterol that may be formed endogenously or absorbed from the diet. Significant amounts of oxysterols have frequently been identified in foods of animal origin, in particular highly processed foods. To date, oxysterols have been shown
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.