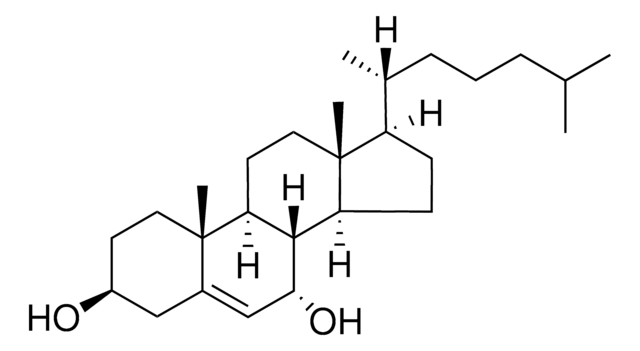
추천 제품
Quality Level
분석
≥98%
양식
powder
배송 상태
ambient
저장 온도
room temp
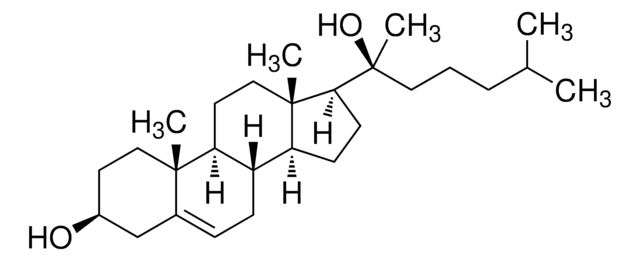
SMILES string
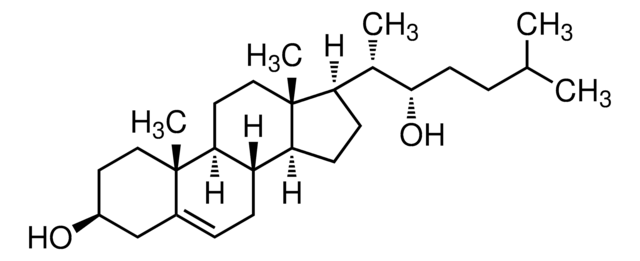
[H][C@@]12CC=C3C[C@@H](O)CC[C@]3(C)[C@@]1([H])CC[C@]4(C)[C@H](CC[C@@]24[H])[C@H](C)[C@H](O)CCC(C)C
InChI
1S/C27H46O2/c1-17(2)6-11-25(29)18(3)22-9-10-23-21-8-7-19-16-20(28)12-14-26(19,4)24(21)13-15-27(22,23)5/h7,17-18,20-25,28-29H,6,8-16H2,1-5H3/t18-,20-,21-,22+,23-,24-,25+,26-,27+/m0/s1
InChI key
RZPAXNJLEKLXNO-GFKLAVDKSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Bovine aortic endothelial cells were treated with 22(R)-Hydroxycholesterol to study the effects on production of free radicals and in studies related to fatty acid metabolism.
생화학적/생리학적 작용
22(R)-Hydroxycholesterol is an intermediate of the pregnenolone synthesis pathway from cholesterol. It reported has neuroprotective properties and protects the neurons against β-amyloid-induced cell death. 22(R)-Hydroxycholesterol acts as the ligand of liver X receptors that act as sensors of sterol concentration and regulates the fatty acid metabolism.
제조 메모
22(R)-Hydroxycholesterol yield clear, colorless solution in chloroform.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
A Chawla et al.
Science (New York, N.Y.), 294(5548), 1866-1870 (2001-12-01)
Cholesterol, fatty acids, fat-soluble vitamins, and other lipids present in our diets are not only nutritionally important but serve as precursors for ligands that bind to receptors in the nucleus. To become biologically active, these lipids must first be absorbed
V Papadopoulos et al.
Journal of neuroendocrinology, 24(1), 93-101 (2011-06-01)
The overall ability of the brain to synthesise neuroactive steroids led us to the identification of compounds that would reproduce aspects of neurosteroid pharmacology. The rate-determining step in neurosteroid biosynthesis is the import of the substrate cholesterol into the mitochondria
Jingsong Ou et al.
Circulation research, 97(11), 1190-1197 (2005-10-15)
Previously we showed L-4F, a novel apolipoprotein A-I (apoA-I) mimetic, improved vasodilation in 2 dissimilar models of vascular disease: hypercholesterolemic LDL receptor-null (Ldlr(-/-)) mice and transgenic sickle cell disease mice. Here we determine the mechanisms by which D-4F improves vasodilation
Zhi-Xing Yao et al.
Journal of neurochemistry, 83(5), 1110-1119 (2002-11-20)
22R-hydroxycholesterol, a steroid intermediate in the pathway of pregnenolone formation from cholesterol, was found at lower levels in Alzheimer's disease (AD) hippocampus and frontal cortex tissue specimens compared to age-matched controls. beta-Amyloid (Abeta) peptide has been shown to be neurotoxic
Lourdes Cruz-Garcia et al.
Comparative biochemistry and physiology. Part A, Molecular & integrative physiology, 160(2), 125-136 (2011-06-04)
The liver X receptor (LXR) has recently been described in salmonids. In mammals, this receptor is already known as a transcriptional factor that regulates diverse aspects of cholesterol, fatty acid and carbohydrate metabolism in various tissues, including muscle. Here we
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.