추천 제품
Grade
technical grade
Quality Level
mp
284 °C (dec.) (lit.)
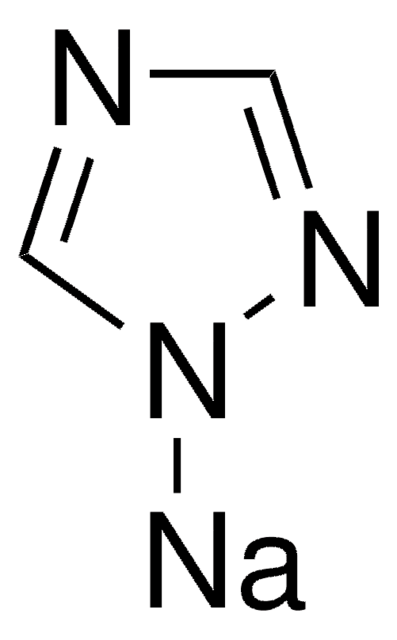
SMILES string
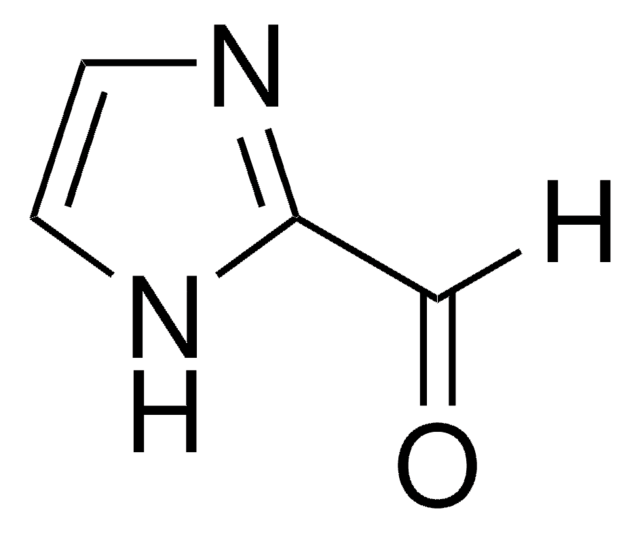
[Na]n1ccnc1
InChI
1S/C3H3N2.Na/c1-2-5-3-4-1;/h1-3H;/q-1;+1
InChI key
ITAWMPSVROAMOE-UHFFFAOYSA-N
애플리케이션
Imidazole sodium derivative (Imidazolylsodium) was used in the synthesis of arylazidoamorphigenin.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
F G Earley et al.
The Biochemical journal, 224(2), 525-534 (1984-12-01)
A photoaffinity-labelling analogue of the respiratory inhibitor rotenone was synthesized from the naturally occurring rotenoid amorphigenin. The analogue inhibits NADH-ubiquinone oxidoreductase activity at concentrations comparable with those of rotenone. Photolysis of the radiolabelled analogue bound to isolated NADH-ubiquinone oxidoreductase resulted
Richard C Knighton et al.
Chemical communications (Cambridge, England), 49(23), 2293-2295 (2013-02-15)
The dansyl fluorophore ligated to gold nanoparticles via imidazole and amine groups affords conjugates capable of detecting micromolar concentrations of the chemical warfare agent sulfur mustard by a fluorescence switching 'ON' displacement assay.
Yin Gao et al.
Bioorganic & medicinal chemistry, 21(5), 1305-1311 (2013-02-05)
Galactosyltransferases (GalTs) extend the glycan chains of mammalian glycoproteins by adding Gal to terminal GlcNAc residues, and thus build the scaffolds for biologically important glycan structures. We have shown that positively charged bivalent imidazolium salts in which the two imidazolium
Chuanjiang Hu et al.
Inorganic chemistry, 52(6), 3170-3177 (2013-03-09)
The effects of the deprotonation of coordinated imidazole on the vibrational dynamics of five-coordinate high-spin iron(II) porphyrinates have been investigated using nuclear resonance vibrational spectroscopy. Two complexes have been studied in detail with both powder and oriented single-crystal measurements. Changes
Ho Young Lee et al.
Proceedings of the National Academy of Sciences of the United States of America, 110(14), 5416-5421 (2013-03-16)
RNA-binding proteins control the fate and function of the transcriptome in all cells. Here we present technology for isolating RNA-protein partners efficiently and accurately using an engineered clustered regularly interspaced short palindromic repeats (CRISPR) endoribonuclease. An inactive version of the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.