모든 사진(1)
About This Item
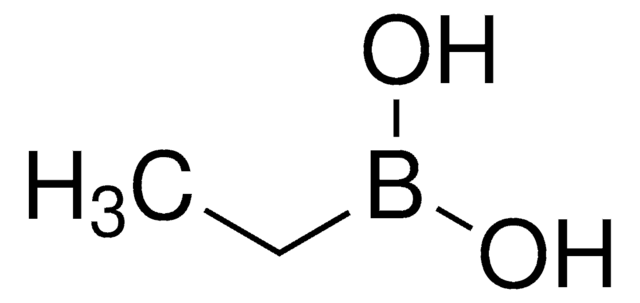
Linear Formula:
CH3B(OH)2
CAS Number:
Molecular Weight:
59.86
Beilstein:
1731087
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
97%
형태
solid
mp
91-94 °C (lit.)
SMILES string
CB(O)O
InChI
1S/CH5BO2/c1-2(3)4/h3-4H,1H3
InChI key
KTMKRRPZPWUYKK-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Methylboronic acid can be used as a reagent:
- In the palladium-catalyzed Stille and Suzuki-Miyaura cross-couplings.
- In the microwave-heated heterogeneous palladium (Pd)-catalytized reactions.
- In ruthenium (Ru)-catalyzed silylation reactions
- To prepare bis(aminotropone) titanium (Ti) catalysts for ethylene polymerizations.
- In the enantioselective asymmetric bromoaminocyclization and bromoaminocyclization using amino-thiocarbamate catalysts.
- To prepare common building blocks for pharmaceuticals and agrochemicals.
- To prepare chrysin analogs by Suzuki-Miyaura coupling reactions.
- To prepare casein kinase I inhibitors.
- In the divergent C-H functionalizations directed by sulfonamide pharmacophores in drug discovery.
- In the synthesis of unsymmetrical monosulfides from disulfides via copper-catalyzed coupling with boronic acids.
- In a palladium-catalyzed coupling with enol tosylates.
- For derivatizing many carbohydrates and biologically active compounds for GLC analysis.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Dietrich Steinhuebel et al.
The Journal of organic chemistry, 70(24), 10124-10127 (2005-11-19)
[reaction: see text] Herein we demonstrate functionalized enol tosylates to be robust substrates that undergo Suzuki-Miyaura, Sonogashira, and Stille cross-coupling reactions to provide stereodefined trisubstituted unsaturated esters.
Ben W Glasspoole et al.
Chemical communications (Cambridge, England), 48(9), 1230-1232 (2011-12-20)
Palladium-catalyzed cross-coupling reactions of secondary allylic boronic esters with iodoarenes were demonstrated under the conditions previously described for the coupling of benzylic substrates. The regioselectivity of the process was largely dictated by the pattern of olefin substitution.
A comparative study of ethylene polymerization by bis(aminotropone) Ti catalysts
Goldani, M. T.; et al.
Polym. Bull., 68, 755-773 (2012)
Ling Zhou et al.
Journal of the American Chemical Society, 132(44), 15474-15476 (2010-10-16)
A novel amino-thiocarbamate-catalyzed bromolactonization of unsaturated carboxylic acids has been developed. The scope of the reaction is evidenced by 22 examples of γ-lactones with up to 99% yield and 93% ee. The protocol was applied in the enantioselective synthesis of
Ling Zhou et al.
Journal of the American Chemical Society, 133(24), 9164-9167 (2011-05-05)
A facile and efficient enantioselective bromoaminocyclization of unsaturated sulfonamides has been developed using an amino-thiocarbamate catalyst. A range of enantioenriched pyrrolidines were prepared with up to 99% yield and 99% ee. The corresponding lactams could be obtained through oxidation of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)