145491
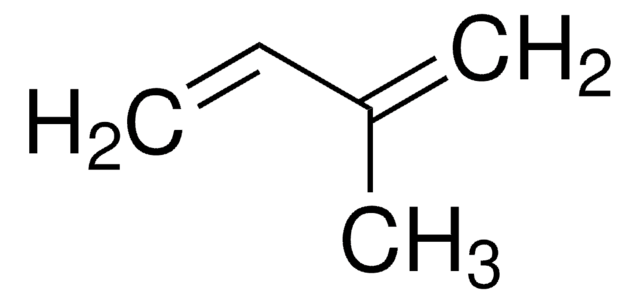
2,3-Dimethyl-1,3-butadiene
98%, contains 100 ppm BHT as stabilizer
동의어(들):
2,3-Dimethylbuta-1,2-diene, 2,3-Dimethylenebutane, Biisopropenyl, Diisopropenyl
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
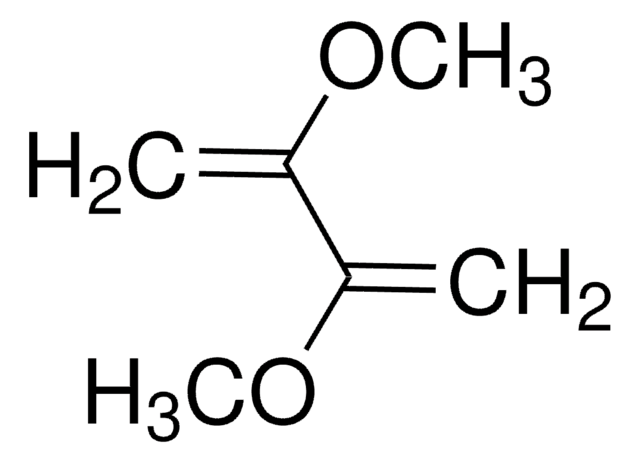
CH2=C(CH3)C(CH3)=CH2
CAS Number:
Molecular Weight:
82.14
Beilstein:
605285
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
vapor pressure
269 mmHg ( 37.7 °C)
분석
98%
양식
liquid
포함
100 ppm BHT as stabilizer
refractive index
n20/D 1.438 (lit.)
bp
68-69 °C (lit.)
mp
−76 °C (lit.)
density
0.726 g/mL at 25 °C (lit.)
저장 온도
2-8°C
SMILES string
CC(=C)C(C)=C
InChI
1S/C6H10/c1-5(2)6(3)4/h1,3H2,2,4H3
InChI key
SDJHPPZKZZWAKF-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
2,3-Dimethyl-1,3-butadiene (DMBD) is a conjugated diene. It undergoes Diels Alder cycloaddition reaction with 2-thio-3-chloroacrylamides under thermal, catalytic and microwave conditions. It undergoes thermal [4+2] cycloaddition reaction with 3-acetyl-, 3-carbamoyl and 3-ethoxycarbonylcoumarins under solvent free conditions. DMBD participates in polymerization reactions in the presence of iron dichloride complexes based catalysts.
애플리케이션
2,3-Dimethyl-1,3-butadiene was used to investigate the reactions of 1,3-dienes with the Si(001) surface using scanning tunneling microscopy and fourier transform infrared spectroscopy.
It may be used in the following processes:
It may be used in the following processes:
- Preparation of 1,3,6-triene derivatives of corresponding 1-aryl-substituted 1,3-dienes by 1,4-hydrobutadienylation in the presence of cobalt catalyst.
- Synthesis of 6-aryl(hetaryl)-3,4-dimethyl-1-nitro-1-cyano-3-cyclohexenes by reacting with gem-cyanonitroethenes.
- As a halogen trap during the study of the photolysis reaction of dibromo adduct of 2,5-diphenyltellurophene.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
30.2 °F - closed cup
Flash Point (°C)
-1 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Polymerization of 1, 3-dienes with iron complexes based catalysts: Influence of the ligand on catalyst activity and stereospecificity.
Ricci G, et al.
J. Mol. Catal. A: Chem., 204, 287-293 (2003)
Cobalt-Catalyzed 1, 4-Hydrobutadienylation of 1-Aryl-1,3-dienes with 2,3-Dimethyl-1,3-butadiene.
Bohn MA, et al.
Angewandte Chemie (International Edition in English), 50(41), 9689-9693 (2011)
Irma Y Flores-Larios et al.
Molecules (Basel, Switzerland), 15(3), 1513-1530 (2010-03-26)
The thermal [4+2] cycloadditions of 3-acetyl-, 3-carbamoyl, and 3-ethoxycarbonylcoumarins with 2,3-dimethyl-1,3-butadiene under solvent free conditions are reported, as well as the epoxidation reactions of some adducts. Discussion is focused on the structural features of the Diels-Alder adducts and their epoxides
Peroxy radical kinetics resulting from the OH-initiated oxidation of 1,3-butadiene, 2,3-dimethyl-1,3-butadiene and isoprene.
Jenkin ME, et al.
Journal of Atmospheric Chemistry, 29(3), 267-298 (1998)
Marie Kissane et al.
Organic & biomolecular chemistry, 8(24), 5602-5613 (2010-10-12)
The Diels-Alder cycloadditions of cyclopentadiene and 2,3-dimethyl-1,3-butadiene to a range of 2-thio-3-chloroacrylamides under thermal, catalytic and microwave conditions is described. The influence of reaction conditions on the outcome of the cycloadditions, in particular the stereoselectivity and reaction efficiency, is discussed.
문서
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.