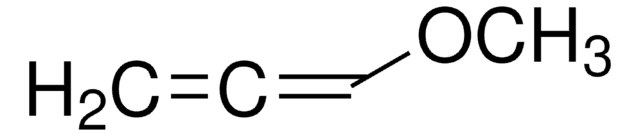모든 사진(1)
About This Item
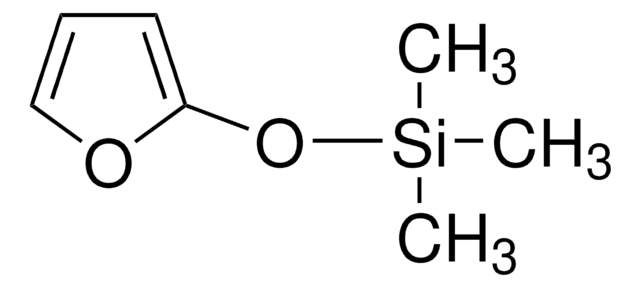
Linear Formula:
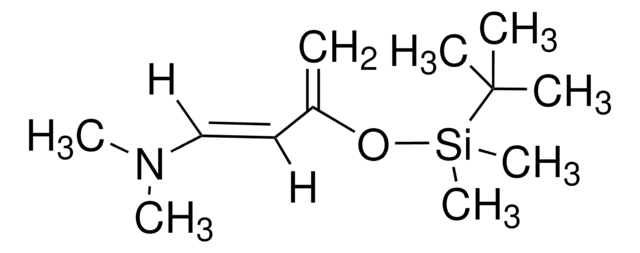
(CH3)3SiOC(=CH2)CH=CHOCH3
CAS Number:
Molecular Weight:
172.30
Beilstein:
1616761
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
95%
양식
liquid
불순물
2-5% 4-methoxy-3-buten-2-one
refractive index
n20/D 1.454 (lit.)
bp
68-69 °C/14 mmHg (lit.)
density
0.885 g/mL at 25 °C (lit.)
작용기
ether
저장 온도
2-8°C
SMILES string
CO\C=C\C(=C)O[Si](C)(C)C
InChI
1S/C8H16O2Si/c1-8(6-7-9-2)10-11(3,4)5/h6-7H,1H2,2-5H3/b7-6+
InChI key
SHALBPKEGDBVKK-VOTSOKGWSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
trans-1-methoxy-3-trimethylsiloxy-1,3-butadiene is a funtionalized Diels-Alder diene. Mukaiyama-Michael-type addition/heterocyclization of trans-1-methoxy-3-trimethylsiloxy-1,3-butadiene (Danishefsky′s diene) with 1,2-diaza-1,3-butadiene has been investigated. Asymmetric hetero-Diels-Alder cyclization of Danishefsky′s diene with benzaldehyde catalyzed by mesoporous inorganic/metalorganic hybrid materials has been reported.
애플리케이션
trans-1-Methoxy-3-trimethylsiloxy-1,3-butadiene was used:
- in the synthesis of sulfone analogues of griseofulvin (sulfogriseofulvins), 4H-1-aminopyrroles and 4,5H-pyrazoles
- as Diels-Alder diene for the synthesis of pyridones and pyranones
- as reagent employed in the Mannich-Michael reaction for preparation of piperidinones and enaminones
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
138.2 °F - closed cup
Flash Point (°C)
59 °C - closed cup
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
이미 열람한 고객
Screening of rare earth metal grafted MCM-41 silica for asymmetric catalysis.
Gerstberger G and Anwander R.
Microporous and Mesoporous Materials : The Official Journal of the International Zeolite Association, 44, 303-310 (2001)
The Journal of Organic Chemistry, 57, 4444-4444 (1992)
Tetrahedron, 49, 397-397 (1993)
Marie-Laure Teyssot et al.
The Journal of organic chemistry, 72(7), 2364-2373 (2007-03-10)
Electron-poor 6-oxo-1-sulfonyl-1,6-dihydropyridine-3-carboxylates 1b-d undergo cross-Diels-Alder reactions with electron-rich dienes 4a-f under hyperbaric conditions, reacting either as dienophiles to yield normal-electron-demand (NED) cycloadducts 10 and/or 11 or as dienes to give inverse-electron-demand (IED) cycloadducts 12 and/or 13. The latter are converted
Orazio A Attanasi et al.
Organic letters, 10(10), 1983-1986 (2008-04-23)
The versatility of the Mukaiyama-Michael-type addition/heterocyclization of Danishefsky's diene with 1,2-diaza-1,3-butadienes was applied to the synthesis of both 4 H-1-aminopyrroles and 4,5 H-pyrazoles. Thus, the same reagents furnished different types of highly functionalized azaheterocycles essentially depending on their structure: as
문서
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.