모든 사진(1)
About This Item
Linear Formula:
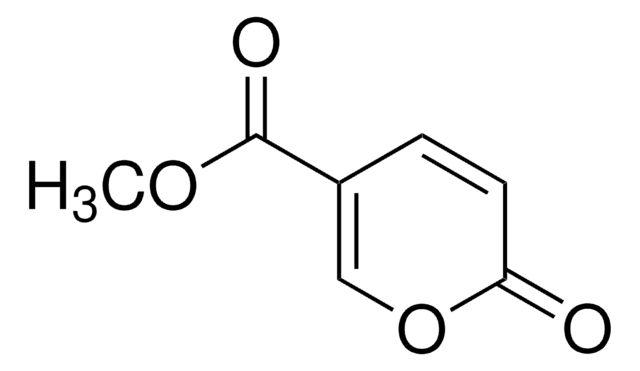
CH2=CHCH=CHOCOCH3
CAS Number:
Molecular Weight:
112.13
Beilstein:
1743394
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
vapor pressure
40 mmHg ( 60 °C)
Quality Level
양식
liquid
포함
0.1% p-tert-butylcatechol as stabilizer
refractive index
n20/D 1.469 (lit.)
bp
60-61 °C/40 mmHg (lit.)
density
0.945 g/mL at 25 °C (lit.)
작용기
ester
저장 온도
2-8°C
SMILES string
CC(=O)O\C=C\C=C
InChI
1S/C6H8O2/c1-3-4-5-8-6(2)7/h3-5H,1H2,2H3/b5-4+
InChI key
NMQQBXHZBNUXGJ-SNAWJCMRSA-N
일반 설명
1-Acetoxy-1,3-butadiene (1-ABD) can be generated by potassium or sodium acetate catalyzed reaction between crotonaldehyde and acetic anhydride. The product is a mixture of cis and trans forms, that has been confirmed by its physical properties and IR spectra. It is reported to be formed as a major product during the acetoxylation of 1,3-butadiene (BD) in gas-phase in the presence of Pd-KOAc (palladium-potassium acetate) catalyst. 1-ABD participates as 2Π or 4Π diene in cycloaddition reactions. Enantioselective Diels Alder reaction of 2-methoxy-5-methyl-1,4-benzoquinone with 1-ABD in the presence of molecular sieves (mesh size:4Å) has been reported.
애플리케이션
1-Acetoxy-1,3-butadiene was used as diene in the following reactions:
It was used for the reaction with dienophiles such as maleimides, a juglone, a butyne-1,4-dione and methyl 2-(4-methylphenyl)-2H-azirine-3-carboxylate and during visible light photocatalysis. It was also used as reactant during intermolecular oxa-Pictet-Spengler cyclization.
- Diels-Alder reaction with ortho-carbazolequinones to yield benzocarbazolequinone.
- Diels-Alder reaction with diethyl ketovinylphosphonate, with and without Lewis acid assistance.
- Diels-Alder reaction with methyl acrylate to yield racemic forms of 2-hydroxy-3-cyclohexenecarboxylic acid.
It was used for the reaction with dienophiles such as maleimides, a juglone, a butyne-1,4-dione and methyl 2-(4-methylphenyl)-2H-azirine-3-carboxylate and during visible light photocatalysis. It was also used as reactant during intermolecular oxa-Pictet-Spengler cyclization.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
91.4 °F - closed cup
Flash Point (°C)
33 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Synthesis and absolute configuration of the isomers of homoisocitric acid (1-hydroxy-1,2,4-butanetricarboxylic acid) and the stereochemistry of lysine biosynthesis.
K Chilina et al.
Biochemistry, 8(7), 2846-2855 (1969-07-01)
Journal of the Chemical Society. Perkin Transactions 1, 1925-1925 (1993)
Muriel Compain-Batissou et al.
Chemical & pharmaceutical bulletin, 52(9), 1114-1116 (2004-09-02)
Oxidation of 2- and 3-hydroxycarbazoles with Frémy's salt gave the corresponding ortho-carbazolequinones. These molecules react as carbodienophiles in Diels-Alder reaction with 1-acetoxy-1,3-butadiene and 1,3-cyclopentadiene to provide the novel benzocarbazolequinone structures 15, 16, 18 and 19.
Gas phase acetoxylation of 1, 3-butadiene over palladium catalysts Part 1: the catalytic activity and structure of Pd-Sb-KOAc catalysts.
Shinohara H.
Applied Catalysis, 10(1), 27-42 (1984)
Intra- and Intermolecular Oxa-Pictet-Spengler Cyclization Strategy for the Enantioselective Synthesis of Deoxy Analogues of (+)-Nanomycin A Methyl Ester, (+)-Eleutherin, (+)-Allo-Eleutherin, and (+)-Thysanone
R. T. Sawant, et al.
European Journal of Organic Chemistry, 23, 4442-4449 (2010)
문서
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.