추천 제품
분석
95%
양식
liquid
refractive index
n20/D 1.459 (lit.)
bp
134-136 °C/745 mmHg (lit.)
mp
19 °C (lit.)
density
0.94 g/mL at 25 °C (lit.)
작용기
ether
저장 온도
2-8°C
SMILES string
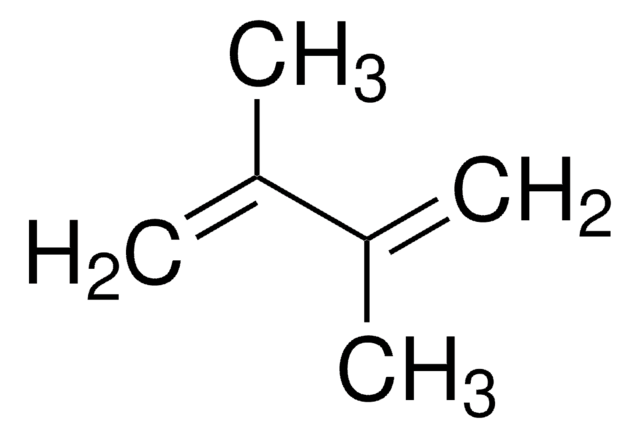
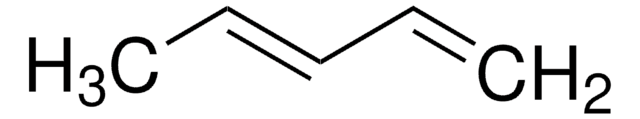
COC(=C)C(=C)OC
InChI
1S/C6H10O2/c1-5(7-3)6(2)8-4/h1-2H2,3-4H3
InChI key
NHBDKDZHQKQPTF-UHFFFAOYSA-N
일반 설명
2,3-Dimethoxy-1,3-butadiene (DMEBD) is a 1,3-butadiene derivative. The reaction kinetics of hydrolysis of 2,3-dimethoxy-1,3-butadiene in the presence of acid catalyst has been investigated. The [4+2] cycloadditions of 3-nitrocoumarins with DMEBD has been investigated in aqueous medium, in organic solvent and under solventless conditions. This reaction led to the formation of 4-substituted 3-nitrochromanones. It forms adducts with graphene and Diels-Alder chemistry in this formation has been investigated.
애플리케이션
2,3-Dimethoxy-1,3-butadiene has been employed as diene to investigate the Diels-Alder chemistry of pristine and defective graphene. It was also used in the synthesis of novel benzopentathiepin varacinium trifluoroacetate.
It may be used in the preparation of 3,4-dimethoxythiophene, an intermediate for the synthesis of 3,4-ethylenedioxythiophene (EDOT). It may also be used to form thio esters by reacting with mercaptans in the presence of cobalt carbonyl catalyst.
It may be used in the preparation of 3,4-dimethoxythiophene, an intermediate for the synthesis of 3,4-ethylenedioxythiophene (EDOT). It may also be used to form thio esters by reacting with mercaptans in the presence of cobalt carbonyl catalyst.
이미 열람한 고객
Influence of dienes on the cobalt carbonyl catalyzed reaction of mercaptans with carbon monoxide.
Antebi S and Alper H.
Organometallics, 5(3), 596-598 (1986)
Santanu Sarkar et al.
Journal of the American Chemical Society, 133(10), 3324-3327 (2011-02-24)
The zero-band-gap electronic structure of graphene enables it to function as either the diene or the dienophile in the Diels-Alder reaction, and this versatile synthetic method offers a powerful strategy for the reversible modification of the electronic properties of graphene
Vinyl ether hydrolysis. XVIII. The two-stage reaction of 2,3-dimethoxy-1,3-butadiene.
Kresge AJ and Yin Y.
Canadian Journal of Chemistry, 65(8), 1753-1756 (1987)
Total synthesis of the novel benzopentathiepin varacinium trifluoroacetate: the viability of" varacin-free base.
Behar V, et al.
Journal of the American Chemical Society, 115(15), 7017-7018 (1993)
David Amantini et al.
The Journal of organic chemistry, 68(24), 9263-9268 (2003-11-25)
The [4 + 2] cycloadditions of 3-nitrocoumarin (1a), 6-chloro-3-nitrocoumarin (1b), and 6-, 7-, and 8-hydroxy-3-nitrocoumarins (1c, 5, and 6) with (E)-piperylene (7), isoprene (8), 2,3-dimethyl-1,3-butadiene (9), 2-methoxy-1,3-butadiene (10), 2,3-dimethoxy-1,3-butadiene (11), and cyclopentadiene (12) were investigated in aqueous medium, in organic
문서
The Diels–Alder reaction is the reaction between a conjugated diene and an alkene (dienophile) to form unsaturated six-membered rings. It is also referred to as a cycloaddition.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.