HPLC Analysis of Pain Relievers on Ascentis® Express C18
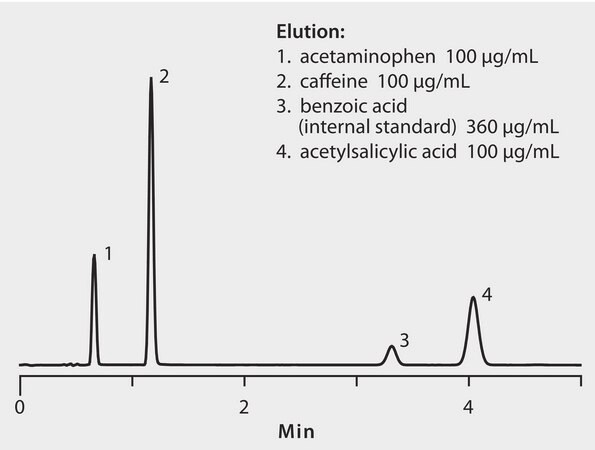
CONDITIONS
column
Ascentis Express C18, 10 cm x 4.6 mm I.D., 5 μm particles (50536-U)
mobile phase
[A] water with 3% acetic acid; [B] methanol with 3% acetic acid; (79:21, A:B)
flow rate
2.0 mL/min
pressure
3290 psi (227 bar)
column temp.
45 °C
detector
UV,275 nm
injection
10 μL
sample
100 - 360 μg/mLin mobile phase
詳細
アナリシスノート
This application shows the improvements in efficiency that may be obtained for USP methods using modern Fused-Core technology.
法的情報
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany