おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
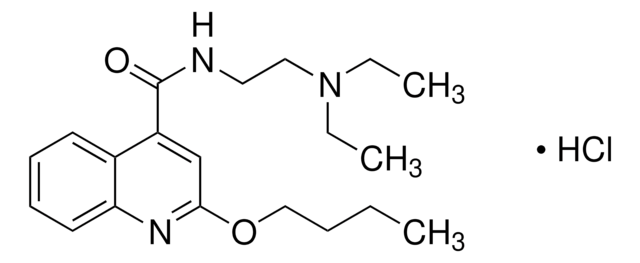
hydromorphone
メーカー/製品名
USP
薬剤管理
USDEA Schedule II
drug control
estupefaciente (Spain); Decreto Lei 15/93: Tabela IA (Portugal)
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
SMILES記法
[N+]1([C@H]2[C@H]3[C@@]4([C@@H](Oc5c4c(ccc5O)C2)C(=O)CC3)CC1)([O-])C
InChI
1S/C17H19NO4/c1-18(21)7-6-17-10-3-5-13(20)16(17)22-15-12(19)4-2-9(14(15)17)8-11(10)18/h2,4,10-11,16,19H,3,5-8H2,1H3/t10-,11+,16-,17-,18?/m0/s1
InChI Key
XDOZFXQPRPKZLY-FQFYJRTASA-N
詳細
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
アプリケーション
Hydromorphone N-oxide hydrochloride USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
アナリシスノート
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
その他情報
Sales restrictions may apply.
関連製品
製品番号
詳細
価格
保管分類コード
11 - Combustible Solids
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)