おすすめの製品
アッセイ
≥98% (HPLC)
形状
powder
色
white to beige
溶解性
DMSO: 2 mg/mL, clear
保管温度
2-8°C
アプリケーション
(Z)-Endoxifen has been used to demonstrate the lack of contribution of subventricular zone and leptomeningeal cells in a study determining the generation of neuroblasts from astrocytes.
生物化学的/生理学的作用
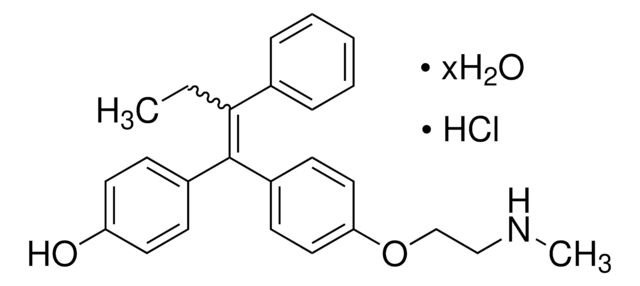
(Z)-Endoxifen (endoxifen) is an active tamoxifen metabolite generated via actions of cytochrome P450 (CYP) enzymes CYP3A4/5 and CYP2D6. Endoxifen is more potent than tamoxifen as a selective estrogen receptor modulator (SERM) both in vitro and in vivo with good pharmacokinetics and oral availability (∼80% MCF-7 tumor growth inhibition with 4-8 mg/kg/day endoxifen or 20 mg/kg/day tamoxifen in mice via p.o.). Endoxifen also exhibits 4-fold higher PKC inhibitory potency than tamoxifen and can overcome tamoxifen resistance due to cytochrome CYP2D6 polymorphism.
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1A - Repr. 1B
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
SML2368-VAR:
SML2368-25MG:
SML2368-5MG:
SML2368-BULK:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Paul Chen et al.
Cellular & molecular biology letters, 23, 3-3 (2018-01-09)
Endoxifen, an active metabolite of tamoxifen, has been shown to be an effective anti-estrogenic agent in estrogen receptor-positive breast cancer patients. In melanoma, estrogen receptor expression is shown to be associated with disease progression. However, the therapeutic benefit of endoxifen
Vered Stearns et al.
Journal of the National Cancer Institute, 95(23), 1758-1764 (2003-12-05)
Tamoxifen, a selective estrogen receptor modulator (SERM), is converted to 4-hydroxy-tamoxifen and other active metabolites by cytochrome P450 (CYP) enzymes. Selective serotonin reuptake inhibitors (SSRIs), which are often prescribed to alleviate tamoxifen-associated hot flashes, can inhibit CYPs. In a prospective
Matthew P Goetz et al.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 35(30), 3391-3400 (2017-08-31)
Purpose Endoxifen is a tamoxifen metabolite with potent antiestrogenic activity. Patients and Methods We performed a phase I study of oral Z-endoxifen to determine its toxicities, maximum tolerated dose (MTD), pharmacokinetics, and clinical activity. Eligibility included endocrine-refractory, estrogen receptor-positive metastatic
Shagufta et al.
European journal of medicinal chemistry, 143, 515-531 (2017-12-06)
Tamoxifen (ICI 46 474), trans-1-(4-β-dimethylaminoethoxyphenyl)-1,2-diphenylbut-1-ene, is the most commonly used drug for the treatment of estrogen receptor positive breast cancer and has been saving lives worldwide for the past four decades. Tamoxifen is considered a pioneering drug due to its ubiquitous
Janina Johänning et al.
Archives of toxicology, 92(3), 1099-1112 (2017-12-30)
Tamoxifen, a standard therapy for breast cancer, is metabolized to compounds with anti-estrogenic as well as estrogen-like action at the estrogen receptor. Little is known about the formation of estrogen-like metabolites and their biological impact. Thus, we characterized the estrogen-like
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)