SML0319
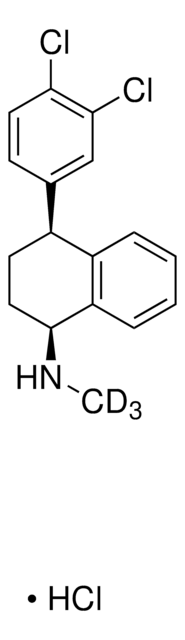
Caramiphen hydrochloride
≥98% (HPLC)
別名:
2-(Diethylamino)ethyl 1-phenylcyclopentane-1-carboxylate hydrochloride, Caramiphenium chloride salt, G 2747, Panparnit, Parpanil, Parpanit, Pentaphen, Pentaphene hydrochloride
About This Item
おすすめの製品
品質水準
アッセイ
≥98% (HPLC)
形状
powder
色
white to beige
溶解性
H2O: ≥5 mg/mL
保管温度
2-8°C
SMILES記法
Cl.CCN(CC)CCOC(=O)C1(CCCC1)c2ccccc2
InChI
1S/C18H27NO2.ClH/c1-3-19(4-2)14-15-21-17(20)18(12-8-9-13-18)16-10-6-5-7-11-16;/h5-7,10-11H,3-4,8-9,12-15H2,1-2H3;1H
InChI Key
MUPNXGNOIBYHSG-UHFFFAOYSA-N
アプリケーション
生物化学的/生理学的作用
特徴および利点
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
SML0319-10MG:
SML0319-50MG:
SML0319-IP:
SML0319-BULK:
SML0319-VAR:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
資料
We offers many products related to muscarinic acetylcholine receptors for your research needs.
Muscarinic acetylcholine receptors are G protein-coupled receptors (GPCRs) and mediate acetylcholine actions in the CNS and non-nervous tissues. Learn more about acetylcholine receptors and their role in cell signaling.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)