すべての画像(2)
About This Item
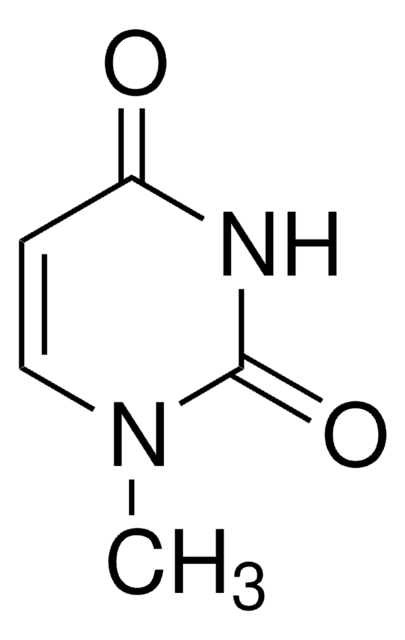
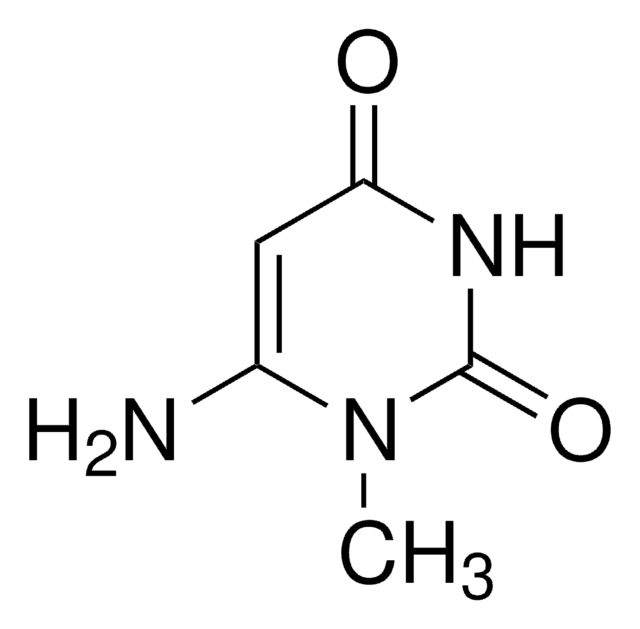
実験式(ヒル表記法):
C5H6N2O2
CAS番号:
分子量:
126.11
MDL番号:
UNSPSCコード:
41106305
PubChem Substance ID:
NACRES:
NA.51
おすすめの製品
アッセイ
≥98% (TLC)
形状
powder
溶解性
1 M NaOH: 50 mg/mL, clear, colorless to faintly yellow
保管温度
−20°C
SMILES記法
CN1C(=O)NC=CC1=O
InChI
1S/C5H6N2O2/c1-7-4(8)2-3-6-5(7)9/h2-3H,1H3,(H,6,9)
InChI Key
VPLZGVOSFFCKFC-UHFFFAOYSA-N
詳細
3-Methyluracil is a methylated uracil or a uracil derivative.
アプリケーション
3-Methyluracil (3-MeU) is used along with other methyl-pyrimidines to study relative physical chemical parameters.
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
M6631-500MG:
M6631-VAR:
M6631-25MG:
M6631-100MG:
M6631-1G:
M6631-BULK:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
T V Chestnova et al.
Bulletin of experimental biology and medicine, 165(6), 777-780 (2018-10-26)
Bacterial biofilms provoke and/or promote the most chronic and recurrent infectious diseases. Previously, experimental models of purulent peritonitis and meningoencephalitis revealed positive antibiofilm effect of metallic nanoparticles and the absence of resistance against such nanoparticles in microorganisms. This study examines
Vibrational Feshbach resonances in uracil and thymine.
Burrow PD, Gallup GA, et al.
J. Chem. Phys. , 28, 124310-124310 (2006)
Mihajlo Etinski et al.
Physical chemistry chemical physics : PCCP, 12(19), 4915-4923 (2010-05-07)
In this work we investigated the lowest-lying electronic excitations for a series of methyl-substituted uracil derivatives, i.e., uracil, 1-methyluracil, 3-methyluracil, thymine, 1-methylthymine, 1,3-dimethyluracil, 3-methylthymine, 1,3-dimethylthymine, and their microhydrated complexes by means of coupled cluster singles and approximate doubles (CC2) and
Guifang Jia et al.
FEBS letters, 582(23-24), 3313-3319 (2008-09-09)
The human obesity susceptibility gene, FTO, encodes a protein that is homologous to the DNA repair AlkB protein. The AlkB family proteins utilize iron(II), alpha-ketoglutarate (alpha-KG) and dioxygen to perform oxidative repair of alkylated nucleobases in DNA and RNA. We
Anna Zhachkina et al.
Journal of the American Chemical Society, 131(51), 18376-18385 (2009-11-26)
The gas-phase substitution reactions of methyl chloride and 1,3-dimethyluracil (at the N1-CH(3)) are examined computationally and experimentally. It is found that, although hydrochloric acid and 3-methyluracil are similar in acidity, the leaving group abilities of chloride and N1-deprotonated 3-methyluracil are
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)